A kind of erythromycin derivative and preparation method thereof
A technology of erythromycin derivatives and halogens, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of low activity of Streptococcus pneumoniae and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
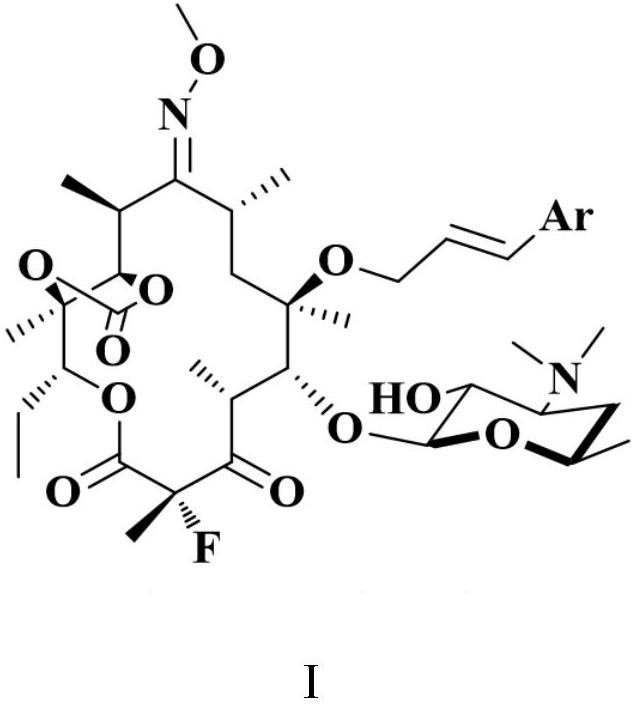
[0049] The present invention provides a kind of erythromycin derivative, and described erythromycin derivative is the compound with general formula I, or, described erythromycin derivative comprises the compound with described general formula I and inorganic acid or organic acid A pharmaceutically acceptable salt formed from an acid, which is metabolized or converted into a compound represented by the general structure as a prodrug under physiological conditions in the body, and plays a pharmacological role as an active ingredient. In the embodiments of the present invention, various pharmaceutically acceptable acids can form salts on the nitrogen of 5-O-desosamine dimethylamino in general formula I, and the prodrug is esterified on the 2'-OH, similarly as Ethyl succinate of erythromycin is used as a prodrug, and the 2'-OH can be released again after hydrolysis of the ester group in vivo. Conventional methods for the preparation of prodrugs can be found in Design of Prodrugs (...
Embodiment 2
[0109] The reaction scheme of an erythromycin derivative in the embodiment of the present invention is as follows, the erythromycin derivative is a compound with general formula I, and the method for preparing the erythromycin derivative may specifically include the following steps:
[0110]
[0111] In order to enable those skilled in the art to better understand the present invention, the preparation method of the erythromycin derivatives in the present invention will be illustrated through a number of specific examples below.
[0112] Step a, the preparation of the second compound (2'-O-acetyl-3-O-descladinose-3-hydroxyl-6-O-allyl erythromycin A9-O-acetyl oxime)
[0113]The dried first compound (2.000 g, 3.170 mmol) was placed in a 100 mL round bottom flask, 20 mL of dry dichloromethane was added, and acetic anhydride (0.900 mL, 9.510 mmol) was added dropwise. The reaction is about 1-1.5h, and the reaction process is monitored by thin-layer chromatography. After the rea...
Embodiment 3
[0229]In vitro antibacterial activity test. The experimental bacteria were sensitive Streptococcus pneumoniae ATCC49619, mef-resistant Streptococcus pneumoniae PU-09, constitutive erm-resistant Streptococcus pneumoniae 07P390, erm+mef-resistant Streptococcus pneumoniae 05O137, induced erm-resistant Streptococcus aureus Coccus PU-32, constitutive erm-resistant Staphylococcus aureus 15B196, inducible erm-resistant Streptococcus pyogenes 01-968, mef-resistant Streptococcus pyogenes 12-207, constitutive erm-resistant Streptococcus pyogenes Streptococcus 12-206, Haemophilus influenzae ATCC49247.
[0230] Experimental method: According to the standard recommended by the Clinical and Laboratory Standards Institute (CLSI, 2010), the broth dilution method was used to measure the in vitro antibacterial activity of some target compounds against the above bacteria. Fresh bacteria were used for the experiment. In each experiment, the standard strain was used as the sensitive experimental...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com