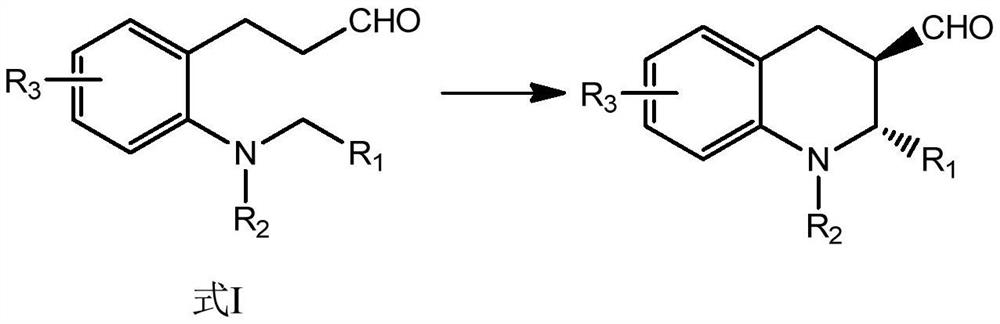
A kind of method utilizing saturated aldehyde to synthesize chiral tetrahydroquinoline
A technology of tetrahydroquinoline and saturated aldehydes, applied in organic chemistry methods, organic chemistry, etc., to achieve high yield and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
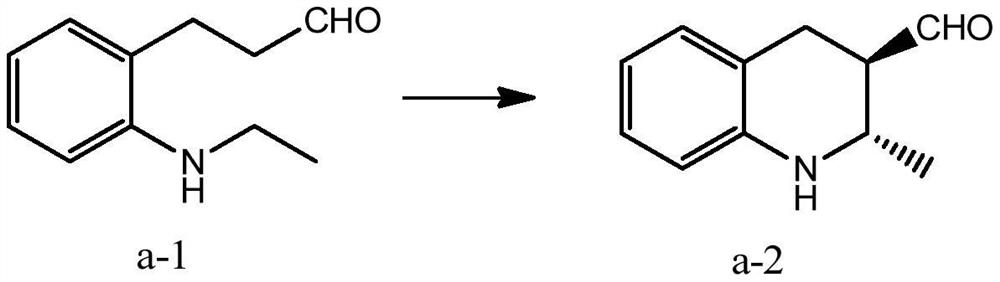
[0043] The present embodiment provides a method for synthesizing chiral tetrahydroquinoline
[0044]
[0045] The mass of 1.00g of compound a-1, 0.37g of diarylprolinol silicon ether, 0.25g of triphenylphosphine rhodium chloride, 0.21g of tri-n-butylamine, at 30 ° C, in 30mL volume ratio of 1 : 1.2 in a mixed solvent of dimethyl sulfoxide and 1,2-dichloroethane, react under an oxygen atmosphere for 80h, filter after the reaction, wash with water, wash with saturated sodium bicarbonate, wash with water again, and extract with dichloromethane, After silica gel column chromatography, 0.81 g of compound a-2 was obtained with a yield of 83.25% and an ee value of 99%.
[0046] 1 H NMR (400MHz, DMSO-d 6 )δ9.74(d,1H),7.14–6.39(m,4H),5.86(s,1H),3.31(p,1H),2.97(ddd,1H),2.81(ddd,1H),2.65–2.34 (m, 1H), 1.00 (d, 3H).
Embodiment 2
[0048] The present embodiment provides a method for synthesizing chiral tetrahydroquinoline
[0049]
[0050] Compound b-1 with a mass of 1.00 g, 0.33 g of diarylprolinol silyl ether, 0.22 g of triphenylphosphine rhodium chloride, 0.18 g of tri-n-butylamine, were dissolved in 40 mL of dimethylmethylene at 20°C. In the sulfone, react under an oxygen atmosphere for 90h, filter after the reaction, wash with water, saturated sodium bicarbonate, and then with water, extract with dichloromethane, and obtain 0.78g of compound b-2 after silica gel column chromatography, with a yield of 79.35%, ee value 98%.
[0051] 1 H NMR (400MHz, DMSO-d 6 )δ10.21–9.85(m,1H),7.19–6.22(m,3H),3.20–2.82(m,3H),2.81–2.52(m,4H),2.22(d,3H),1.09(d, 3H).
Embodiment 3
[0053] The present embodiment provides a method for synthesizing chiral tetrahydroquinoline
[0054]
[0055] Compound c-1 with a mass of 1.00 g, 0.24 g of diarylprolinol silyl ether, 0.35 g of palladium acetate, and 0.25 g of tri-n-butylamine were reacted in 30 mL of dichloromethane at 50 °C under an oxygen atmosphere 100h, filter after the reaction, wash with water, saturated sodium bicarbonate, and water again, extract with dichloromethane, and obtain 0.76 g of compound c-2 after silica gel column chromatography, yield 76.35%, ee value 98%.
[0056] 1 H NMR (400MHz, DMSO-d 6 )δ9.77(d,1H),6.86–6.36(m,2H),3.02(ddd,1H),2.88–2.45(m,6H),2.25(dd,6H),1.88(ddt,1H),1.41 (ddt, 1H), 0.79 (t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com