Immunosuppression-reverting oligonucleotides inhibiting the expression of cd39
An oligonucleotide and immunosuppressive technology, applied in the field of pharmaceutical compositions of excipients and/or diluents, can solve problems such as lack of enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
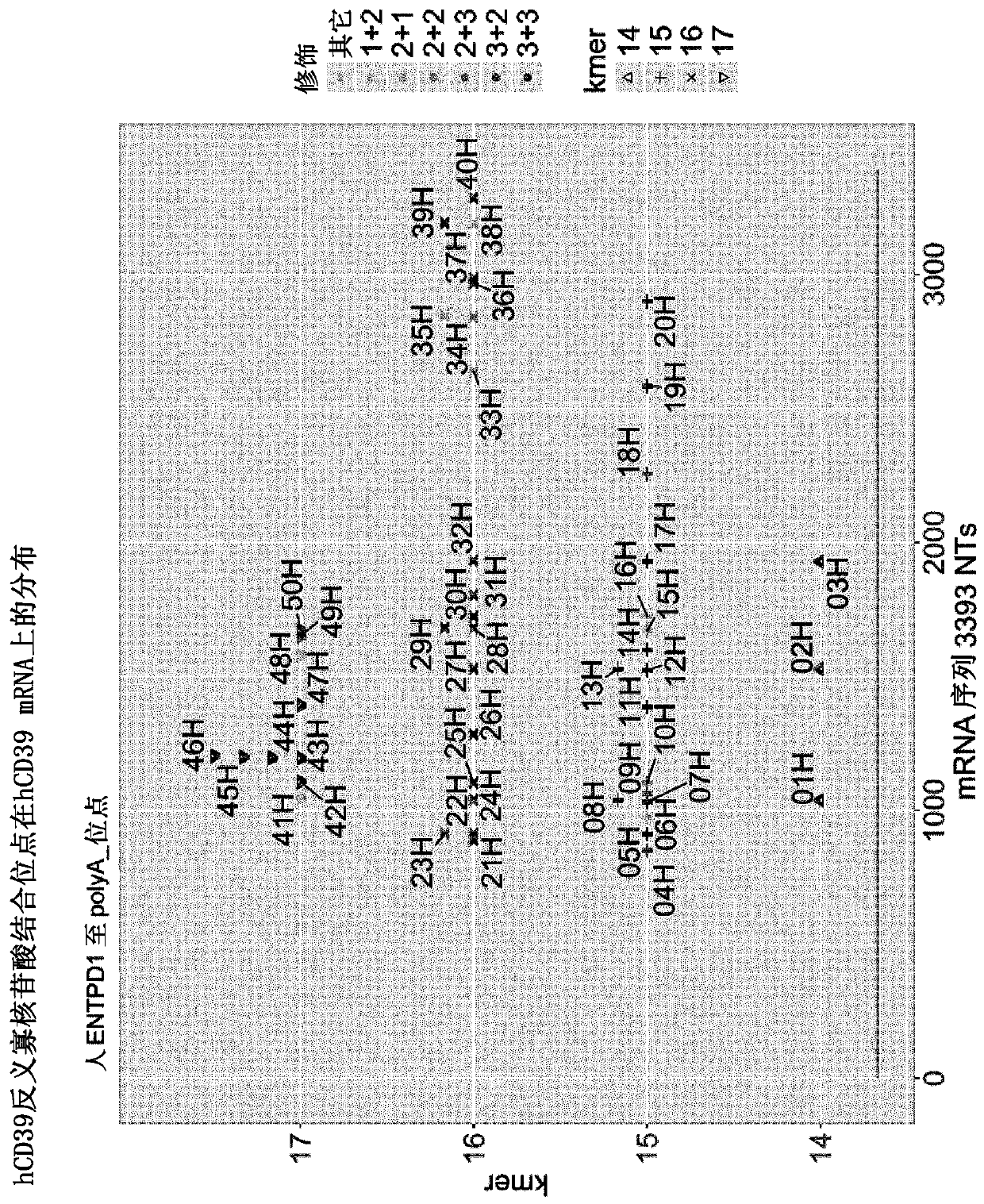
[0069] Embodiment 1: the design of human CD39 antisense oligonucleotide
[0070] For the design of antisense oligonucleotides specific for human (h)CD39, the hCD39 mRNA sequence with SEQ ID No. 1 (sequence reference ID NM_001776.5) was used. 14, 15, 16 and 17mers were designed according to internal standards, neg1 (described in WO2014154843 A1 ) was used as control antisense oligonucleotide in all experiments (Table 1). The distribution of antisense oligonucleotide binding sites on hCD39 mRNA is as follows: figure 1 shown.
Embodiment 2
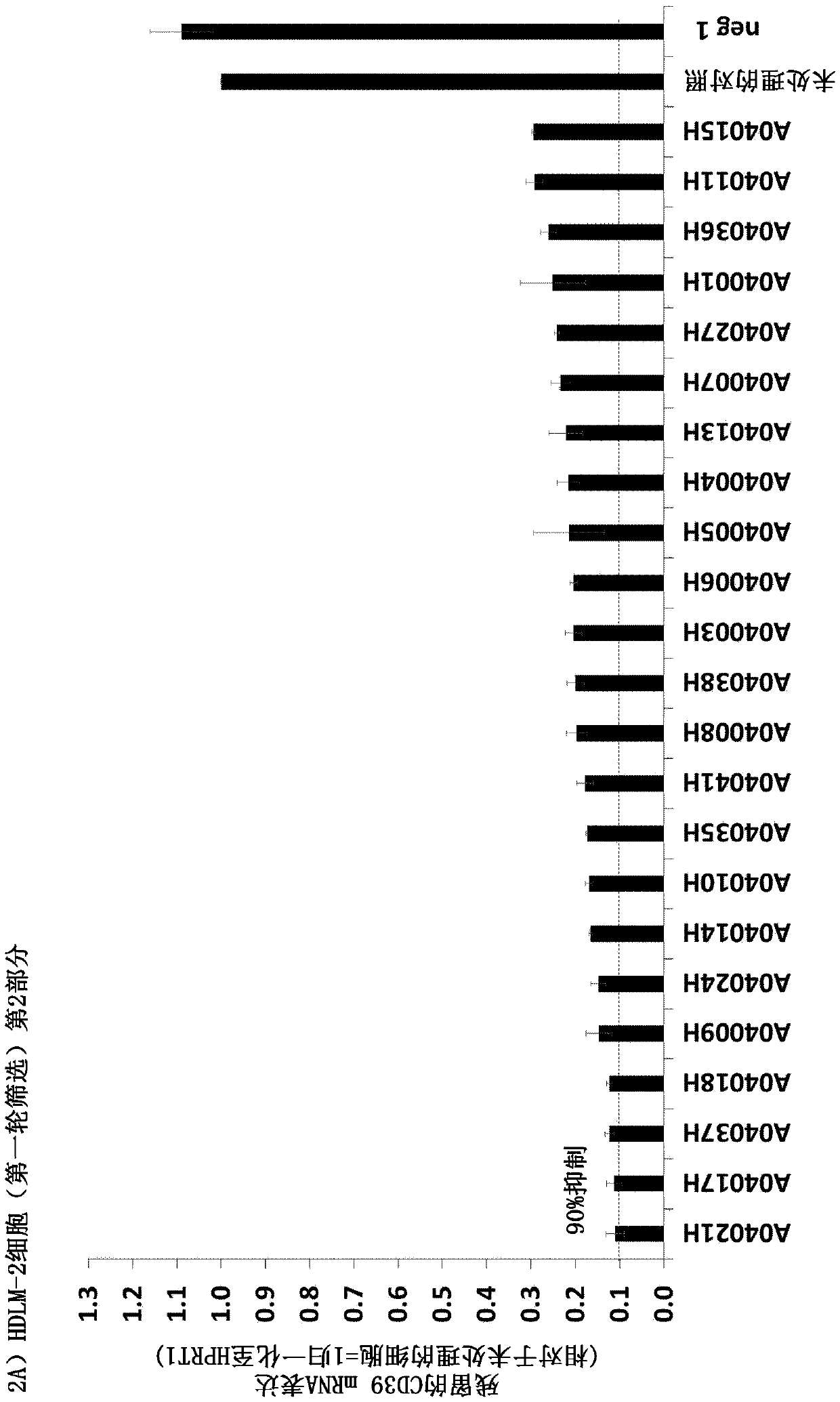
[0071] Example 2: Efficacy screening of hCD39 antisense oligonucleotides in human cancer cell lines
[0072] In order to analyze the efficacy of the hCD39 antisense oligonucleotides of the present invention on the knockdown of hCD39 mRNA expression in cancer cell lines, HDLM- 2 (human Hodgkin's lymphoma, DSMZ) and A-172 (human glioblastoma, ATCC) cells (concentration: 10 μM, without adding any transfection reagent; this process is called autonomous delivery). hCD39 and HPRT1 mRNA expression was analyzed three days later using the QuantiGene Singleplex assay (Affymetrix), and hCD39 expression values were normalized to HPRT1 values. Strikingly, knockout efficiencies of >90% were observed for 23 and 18 (HDLM-2 cells; see Figures 2A and 2B ), and for 8 and 10 (A-172 cells) antisense oligonucleotides. to >90% knockout efficiency (see Figures 2C and 2D). Listed below for A-172 (Table 4 is the first round of screening and Table 5 is the second round of screening) and HDLM-2 cells...
Embodiment 3
[0086] Example 3: Correlation analysis of antisense oligonucleotide efficacy in HDLM-2 and A-172 cells
[0087] To further select the candidate with the highest activity in the two tested cell lines HDLM-2 and A-172, a correlation analysis was performed (data from Figures 2B and 2D). Such as image 3 As indicated, seven effective antisense oligonucleotides were selected for the determination of IC in HDLM-2 and A-172 cells 50 , namely A04019H (SEQ ID No.23), A04033H (SEQ ID No.37), A04039H (SEQ ID No.43), A04040H (SEQ ID No.3), A04042H (SEQ ID No.45), A04044H (SEQ ID No. 47) and A04045H (SEQ ID No. 4) (marked in black). Importantly, the control antisense oligonucleotide neg1 had no negative effect on hCD39 expression in both cell lines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com