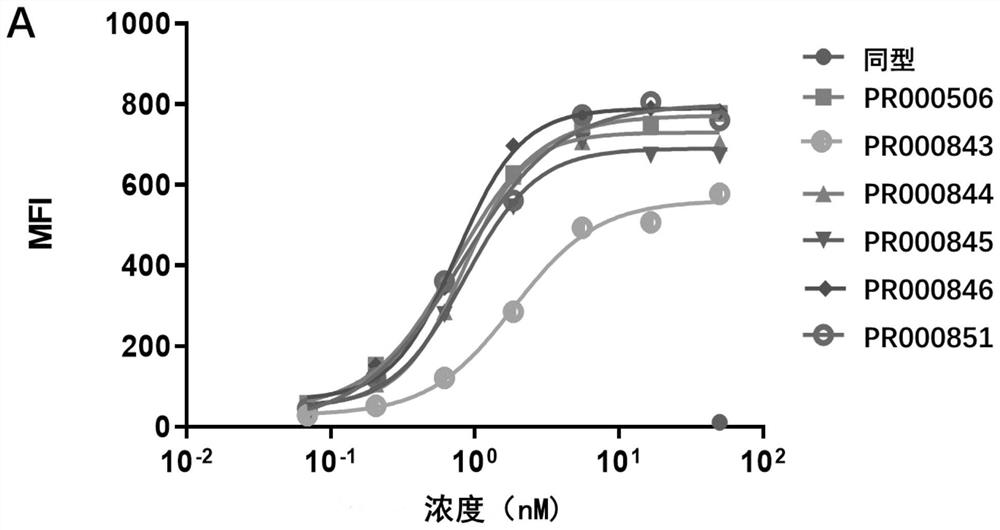
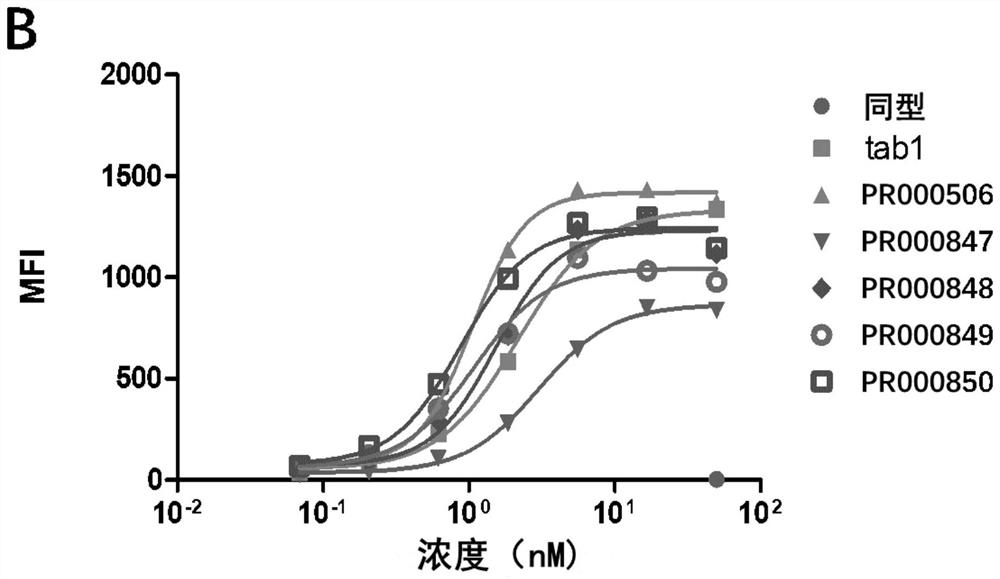
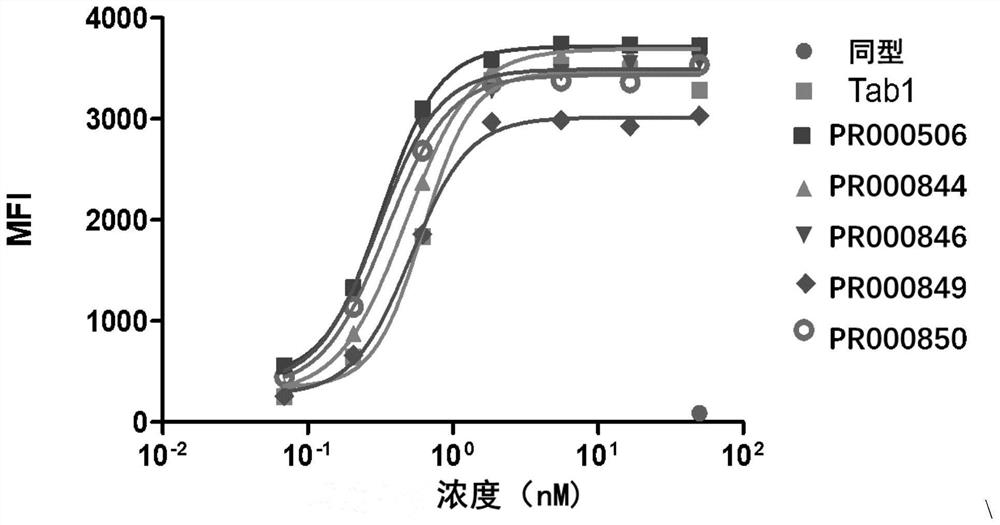
Anti-cd73 antibody and application thereof
An antibody and antibody heavy chain technology, applied in the direction of antibodies, antibody medical components, anti-tumor drugs, etc., can solve the problem that the characteristics of anti-CD73 antibodies are yet to be determined.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0423] Example 1: Production of anti-CD73 antibodies
[0424] immunity
[0425] In immunization using Harbor H2L2 mice, CD73 protein was used as immunogen to generate anti-CD73 antibodies. Each mouse was given a first boost of 50 μg protein followed by a subsequent boost of 25 μg protein using adjuvant (Sigma, S6322) by subcutaneous and intraperitoneal injection. This immunization was performed every two weeks for a total of 6 times. Final immunization was performed by intraperitoneal injection of immunogen diluted in PBS. Serum titers against human CD73 were tested using ELISA and FACS. At indicated time points, mouse sera were sampled and titrated for ELISA and FACS analysis to test for binding to CD73 or CD73 stable cell lines. Good serum titers were observed in the protein-immunized mice.
[0426] hybridoma
[0427] Mouse splenocytes isolated from immunized mice were fused with the mouse myeloma cell line SP2 / 0 (ATCC, CRL-1581) by an electric field-based electroporator...
Embodiment 2
[0428] Example 2 Antibody Production and Purification
[0429] Recombinant plasmids encoding the antibodies of interest were transiently co-transfected into HEK293-6E or 293-F cell cultures using PEI (Polyscience, 24885). 30 μg of plasmid was mixed with 120 μL of PEI and incubated for 15 minutes at room temperature. Next, the mixture was added dropwise at 1 x 10 6 The concentration of cells / ml was suspended in 293 cells in Opti-MEM. After transfection, the cells were cultured on a shaker at 120 rpm at 37° C. and 5% CO 2 . Cell culture supernatants collected on days 6-7 were used for purification.
[0430] The supernatant containing the antibody of interest was harvested 6-7 days after transfection by centrifugation and filtration. Monoclonal antibodies were purified by passing rProtein A (GE, 17-1279-02) prepacked columns (Bio-Rad, 7311550). By packing 0.2 mL of rProteinA resin into a column, followed by 10 column volumes of ddH 2 Prepare the Protein A column by washing ...
Embodiment 3
[0431] Example 3 Antibody Engineering
[0432] The VH and VL sequences of the anti-CD73 antibody PR000506 were further optimized by germlineization and PTM removal operations.
[0433] In the germlineization procedure, the antibody VH or VL sequence is first aligned to the closest human germline sequence by an algorithm such as NCBI / Ig-BLAST, and then the residues in the framework regions that are different from the germline sequence are reversed to Corresponding residues in the germline sequence. Then, germlined antibodies composed of sequence variants are recombinantly produced by mature molecular biology techniques.
[0434] Post-translational modifications (PTMs) are widely observed in proteins expressed in mammalian cells. In addition to the PTM sites conserved in antibodies (such as the N-glycosylation conserved sites on the CH2 domain of IgG1 antibodies), other PTM sites that occur within the antibody antigen-binding site (ie, the CDR region) may reduce the antigen B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com