A kind of polypeptide for ptp1b detection and fluorescent probe comprising the polypeptide
A technology for synthesizing fluorescent probes and solid-phase peptides, applied in the field of fluorescent probes, can solve the problems of large background interference, compound structure influence, and high detection environment requirements in the chromogenic substrate method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 has the fluorescent probe synthesis of AIE characteristic
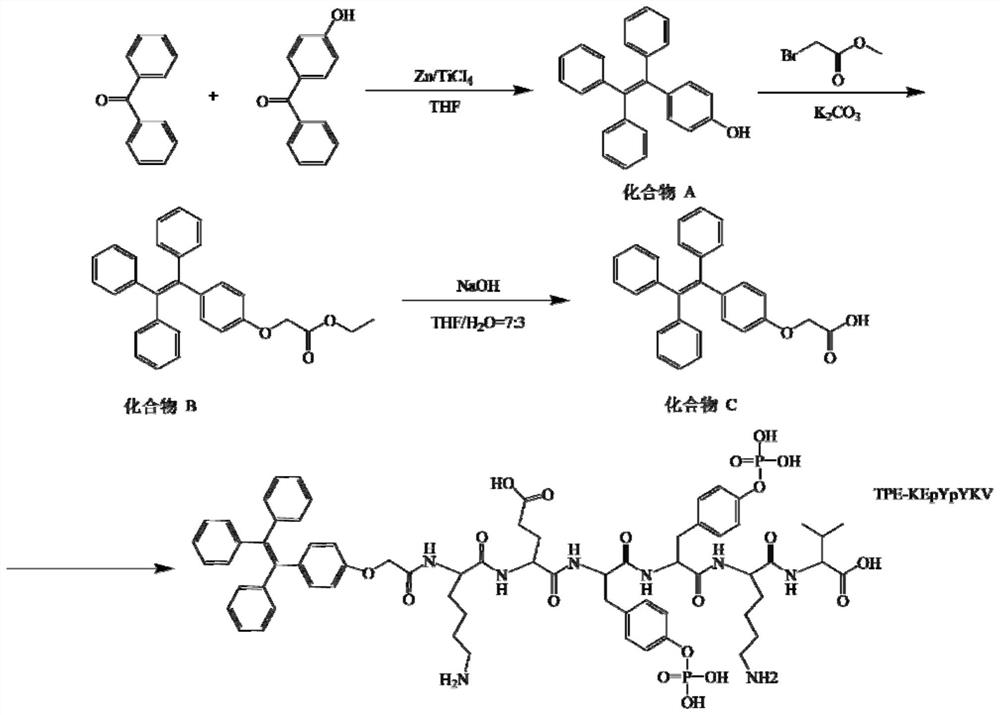
[0046] This embodiment provides a method for synthesizing a fluorescent probe with AIE characteristics, and its synthesis process is as follows figure 1 shown, including:
[0047] (1) Separately synthesize a polypeptide chain through a solid-phase polypeptide synthesis reaction, and the sequence of the polypeptide chain is lysine-glutamic acid-phosphorylated tyrosine-phosphorylated tyrosine-lysine-valine;
[0048] (2) Add 4-hydroxybenzophenone (1.9g, 10mmol), benzophenone (2.2g, 12mmol) and zinc powder (2.9g, 44mmol) into a 250ml three-necked flask, pump air, nitrogen, and repeat three times; Add 80ml of THF (tetrahydrofuran), 0 ℃ ice-water bath for 30min; dropwise add titanium tetrachloride (2.4ml, 22mmol) under ice-water bath, reflux overnight, spin dry; add appropriate amount of dichloromethane and dilute hydrochloric acid for extraction, remove the organic layer, dried with anhydrous magnesiu...
Embodiment 2
[0052] Example 2 Application of Fluorescent Probe TPE-KEpYpYKV in PTP1B Activity Detection
[0053] (1) Fluorescence spectrum analysis of the fluorescent probe and the product after the reaction between the fluorescent probe and the enzyme.
[0054] Sample group 1: Add 50 μL of fluorescent probe TPE-KEpYpYKV with a concentration of 100 μM;
[0055] Sample group 2: Add 1.5 μL of PTP1B with a concentration of 40 μg / mL, 50 μL of fluorescent probe TPE-KEpYpYKV with a concentration of 100 μM;
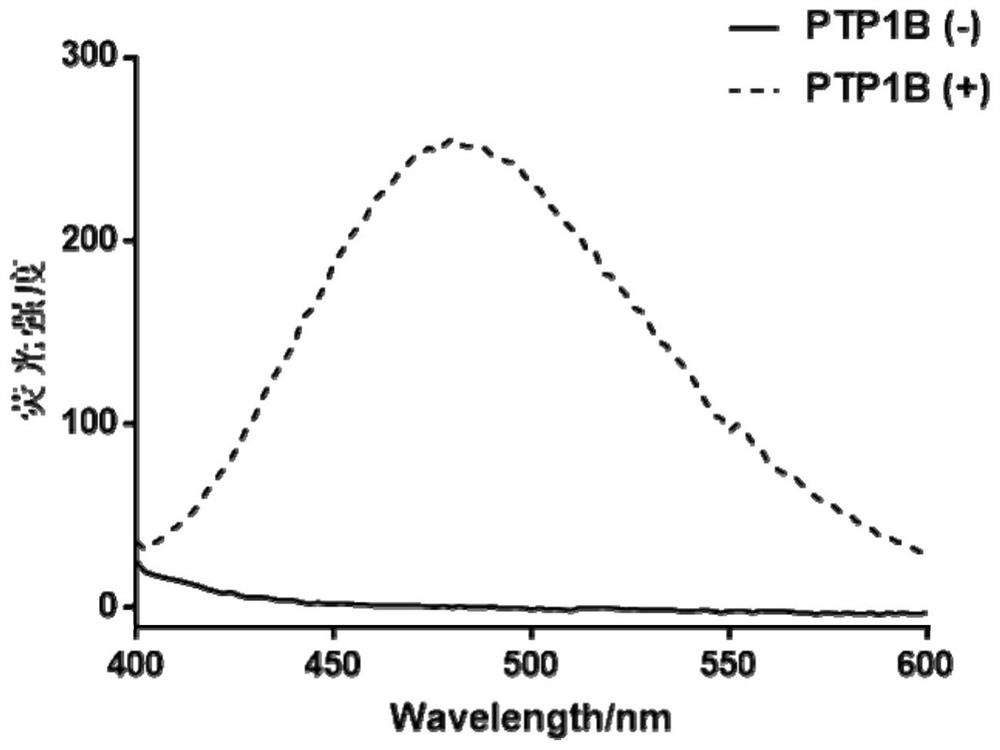
[0056] The two sample groups were buffered with Tris-HCl (10mM, pH 7.5, containing 50mM Na + , 2mMDTT) to 100 μL, and incubated at 37°C for 15 minutes. After the reaction, a Tecan microplate reader was used to measure the fluorescence spectrum from 400 nm to 600 nm under 320 nm excitation.
[0057] See the test results figure 2 .
[0058] Depend on figure 2 It can be seen that the synthesized fluorescent probe TPE-KEpYpYKV basically has no fluorescence absorption in the range of 400-...
Embodiment 3
[0063] Example 3 Application of Fluorescent Probe TPE-KEpYpYKV in Screening PTP1B Inhibitors
[0064] (1) Application of fluorescent probe TPE-KEpYpYKV in screening PTP1B inhibitors
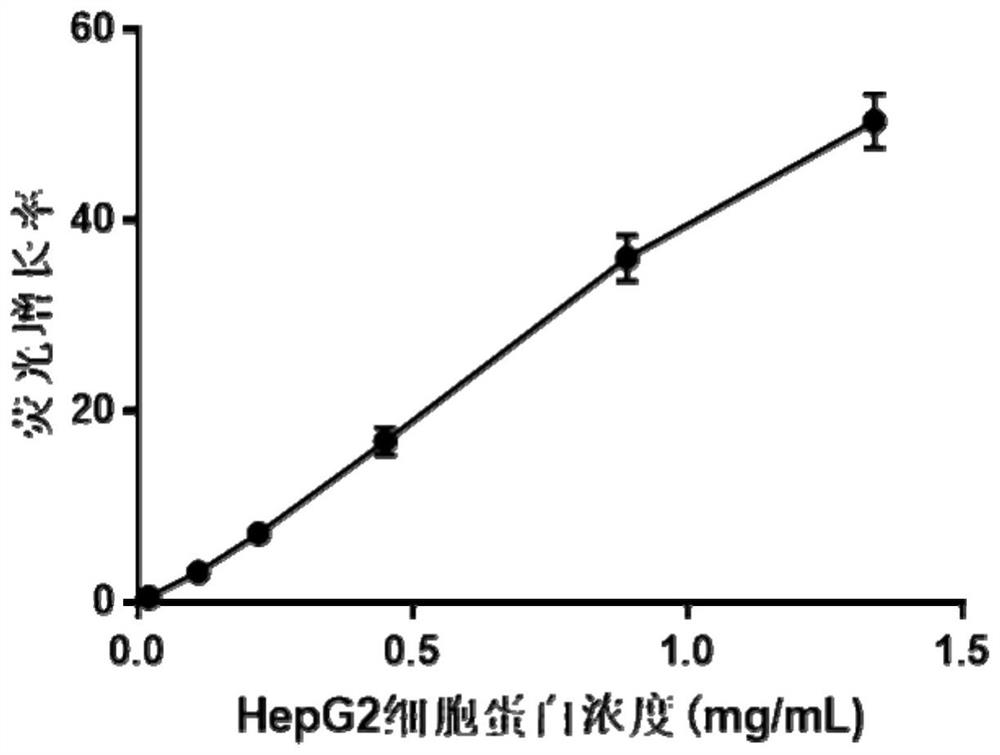
[0065] Take 50 μL of fluorescent probe TPE-KEpYpYKV with a concentration of 100 μM, add 1.5 μL of PTP1B with a concentration of 40 μg / mL and a certain volume of different concentrations of Na 3 VO 4 Mother liquor (making Na 3 VO 4 The final concentration is 0.5, 1, 5, 25, 125, 250, 500μM), with buffer Tris-HCl (10mM, pH 7.5, containing 50mMNa + , 2mMDTT) to 100μL, incubate at 37°C for 15min, measure with a Tecan microplate reader, set E x 320nm (±5nm), E m 480nm (±5nm).
[0066] See the test results Figure 5 .
[0067] Depend on Figure 5 It can be seen that with Na 3 VO 4 Concentration increases, Na 3 VO 4 The inhibitory effect on PTP1B also gradually increased; indicating that the fluorescence growth rate of the fluorescent probe TPE-KEpYpYKV can better reflect the inhibitory effe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com