An active targeting amphiphilic polypeptide nano drug carrier and its preparation and application
An active targeting and nanocarrier technology, applied in the field of biomedicine, can solve the problems of inability to detect tumor sites, low encapsulation efficiency, poor tumor cell targeting, etc., achieving in vivo stability and broad application prospects. , the effect of high packing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation method of active targeting amphiphilic polypeptide nanocarrier
[0044] (i) Preparation of amphiphilic polypeptide molecules:
[0045] (1) Take 0.3 grams of Rink Amide-AM resin to the peptide synthesis device, add dry N,N-dimethylformamide to soak the resin for 2 hours to make it fully swell, and finally discharge the solvent N,N-dimethylformamide . Then use piperidine:N,N-dimethylformamide solution (10 mL) with a volume ratio of 1:4 to remove the protective group of the resin, and react twice for 20 minutes each time. Then wash the resin with 10mL N,N-dimethylformamide repeatedly for 3 times, each time for 5 minutes, take a little resin and add it to the ethanol solution of ninhydrin and phenol, heat to boiling, observe the color change of the resin, if The resin turns blue or even black, indicating that the protective group of the resin has been successfully removed, and the coupling of the first amino acid can be carried out. If the color of ...
Embodiment 8
[0057] Example 8: Preparation method of active targeting amphiphilic polypeptide nano-drug carrier loaded with anti-tumor drugs
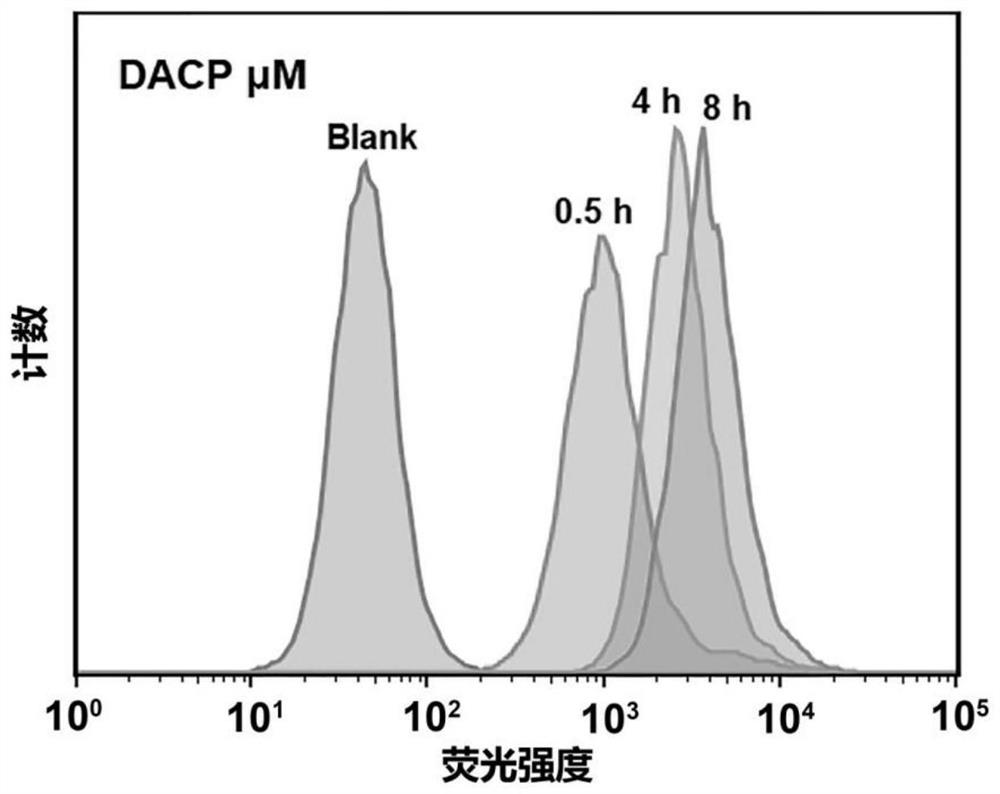
[0058] (i) Preparation of an amphiphilic polypeptide molecule (DACP) containing two hydrophobic alkyl chains: refer to steps (1)-(9) in Example 1.
[0059] (ii) Preparation of nano-drug carrier: at room temperature, according to the mass ratio of the drug to the amphiphilic polypeptide molecule being 1: (2-10), 5 mg of the amphiphilic polypeptide molecule and the hydrophobic antineoplastic drug camptothecin were dissolved in In 170 microliters of dichloromethane solution, remove the dichloromethane solution by rotary evaporation, disperse it in water under ultrasonic conditions, sonicate for 30 minutes, filter the membrane to remove unencapsulated chemotherapeutic drugs, and obtain the product.
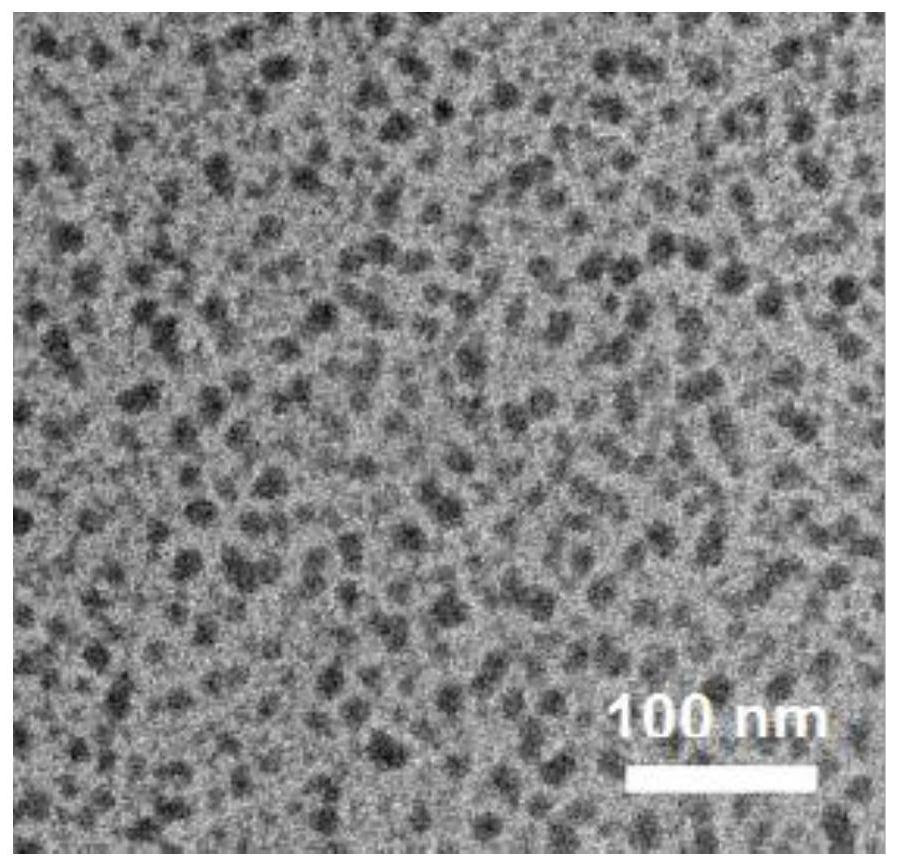
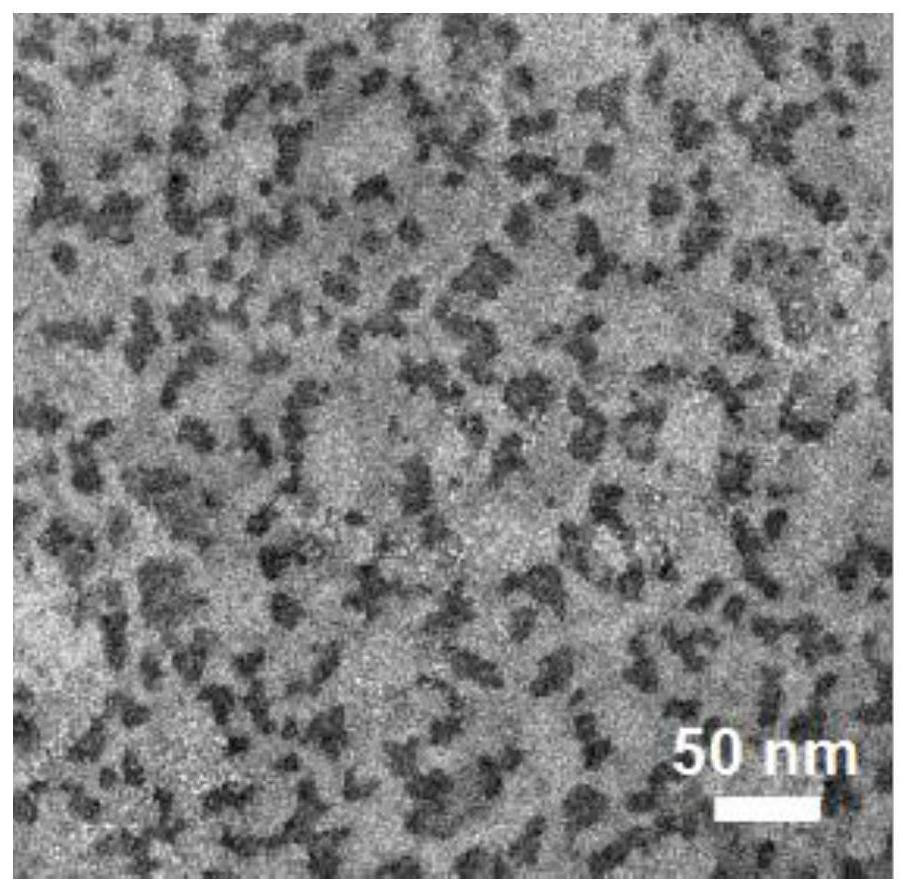
[0060] Morphological observation
[0061] (1) Observing the DACP nano-carrier with an electron microscope, it was found that the prepared nano-carrier was...
Embodiment 9
[0063] Embodiment 9: Determination of Encapsulation Efficiency of Nanocarriers of the Present Invention
[0064] (1) Draw the standard working curve, take by weighing 2.5mg camptothecin and dissolve it in dichloromethane, equipotentially dilute 7 concentrations, each concentration is 1mL, utilize ultraviolet spectrophotometer to measure camptothecin in dichloromethane in ultraviolet Absorbance at the absorption peak; draw a standard working curve.
[0065] (2) The aqueous solution of the nano drug carrier (DACP@CPT NPs) in Example 5 was lyophilized, then dissolved in dichloromethane, and the absorbance value of the antitumor drug in the dichloromethane solution was measured by an ultraviolet spectrophotometer;
[0066] (3) Bring the absorbance value measured in step (2) into the standard working curve, and by calculation, obtain the entrapped mass of camptothecin;
[0067] (4) Calculate the encapsulation efficiency of camptothecin by nanocarriers according to the concentratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com