Active targeting amphiphilic polypeptide composite nanomicelle prodrug and its preparation and application
A nanomicelle and active targeting technology, applied in the field of biomedicine, can solve the problems of poor deep delivery of chemotherapy drugs, inability to track drug distribution in real time, poor targeting of tumor cells, etc. The effect of promoting disassembly and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation method of active targeting amphiphilic composite polypeptide nano-therapeutic agent based on electrostatic interaction
[0046] (i) Preparation of Active Targeting Negatively Charged Amphiphilic Peptides
[0047] (1) Take 0.305 grams of Rink Amide-AM resin to the peptide synthesis device, add dry N,N-dimethylformamide to soak the resin for 2 hours to make it fully swell, and finally discharge the solvent N,N-dimethylformamide . Then, a piperidine:N,N-dimethylformyl solution (10 ml) with a volume ratio of 1:4 was used to remove the protective group of the resin, and the reaction was performed twice, each lasting 20 minutes. Then wash the resin N,N-dimethylformyl repeatedly with 10ml N,N-dimethylformyl for 3 times, each time for 5 minutes, take a little resin and add it to the ethanol solution of ninhydrin and phenol, heat After boiling, observe the color change of the resin. If the resin turns blue or even black, it means that the protective grou...
Embodiment 2
[0064] Embodiment 2: synthetic polypeptide chemotherapeutic prodrug
[0065] Dissolve 1.5g (6.8mmol) of dithiodipyridine in 12ml of ethanol, add 0.16ml of acetic acid into a 50ml two-necked round-bottomed flask, dissolve 235.2μL (312.8mg 3.4mmol) of thioglycolic acid in 8mL of ethanol, and add constant pressure drops In the funnel, under the protection of argon, thioglycolic acid ethanol solution was added dropwise, and stirred at room temperature for 3 h. After the reaction, the solvent was spin-dried to obtain a yellow oily liquid. Purification by high-pressure preparative column yielded pure pyridine acetic acid.
[0066] Take 20.1mg (0.1mmol) of pyridineacetic acid and dissolve it in 400μl DMF, add Pybop 104.1mg (0.2mmol activated carboxyl group) and stir at room temperature for 30min under the protection of argon, take DOX 48.3mg (0.083mmol) and dissolve it in 1.5ml DMF, Add dropwise under air protection, then add DIPEA 69.8μl (0.4mmol 51.7mg), and stir at room temperatu...
Embodiment 3
[0068] Example 3: Preparation of Polypeptide Composite Nanomicelles
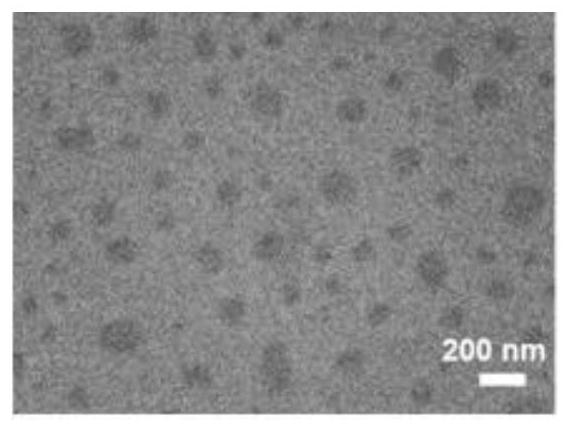
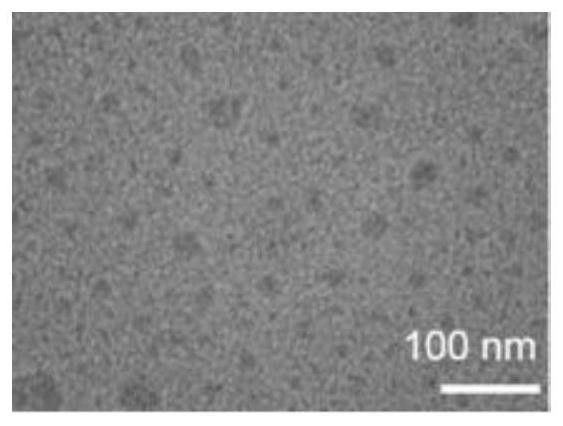
[0069] At room temperature, dissolve in 10 μl DMSO according to the amount ratio of negatively charged polypeptide 1 with active targeting function and positively charged prodrug polypeptide 2: (0.1-5), and then dissolve the peptide containing polypeptide 1 and polypeptide 2 DMSO solution was added to 1ml of PBS while sonicating, then sonicated for 20 minutes, and then dialyzed for 12 hours to obtain polypeptide composite nanomicelles, named PP. figure 1 It is a schematic diagram of the composite nanomicelle. The chain segment outside the sphere is the hydrophilic part of polypeptide 1 and polypeptide 2, the shell is the hydrophobic alkyl chain, the four-pointed star in the hydrophobic cavity is the near-infrared dye, and the sphere is the chemotherapeutic drug DOX.
[0070] Morphological observation: Observing the polypeptide composite nano-therapeutic agent with a transmission electron microscope, it was f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com