Tumor-associated autoantibody and tumor marker combined detection kit
A technology of tumor markers and autoantibodies, which is applied in the field of combined detection kits for tumor-related autoantibodies and tumor markers, can solve the problems of patients missing early detection and treatment, tumor marker levels not significantly increased, and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
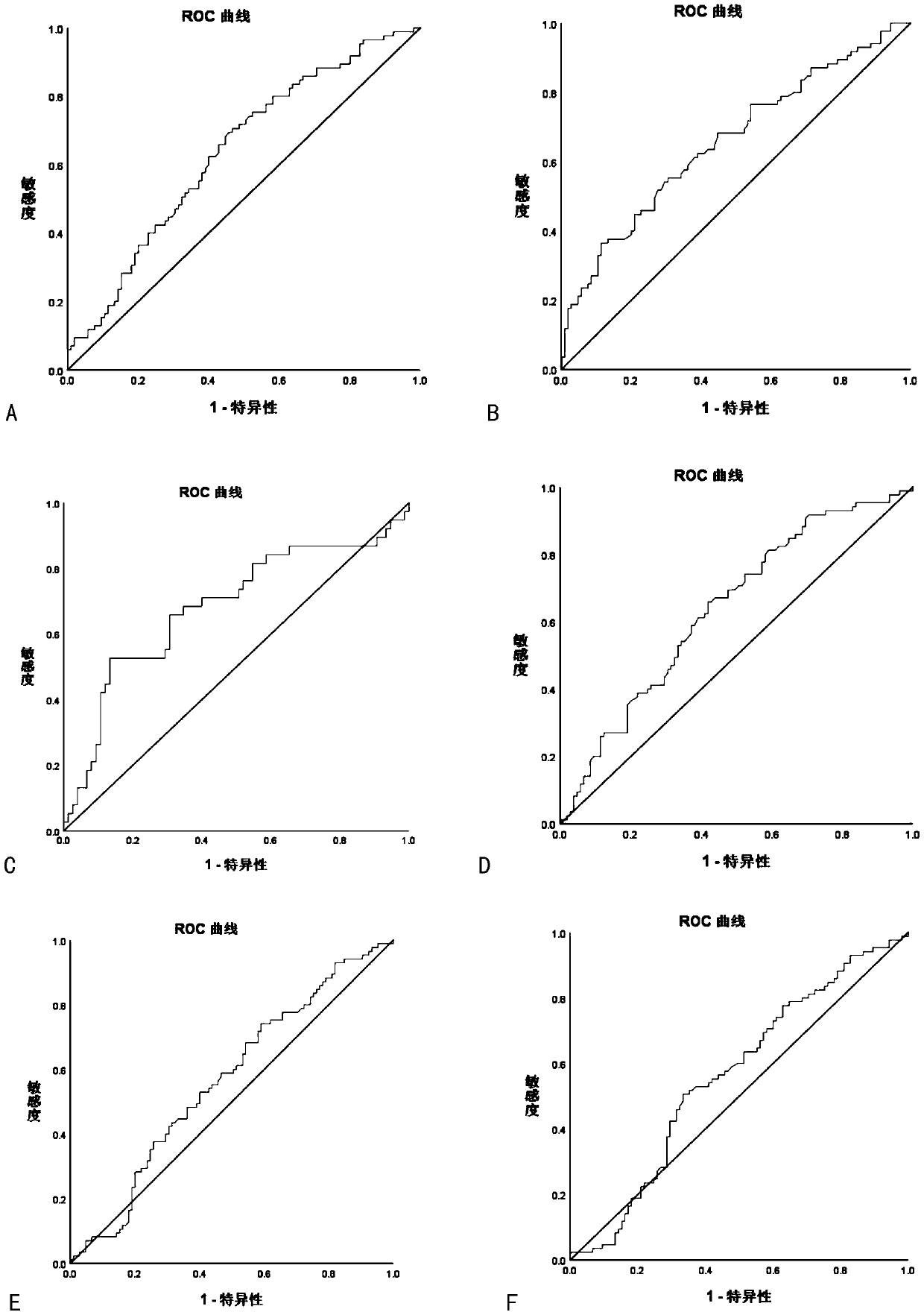
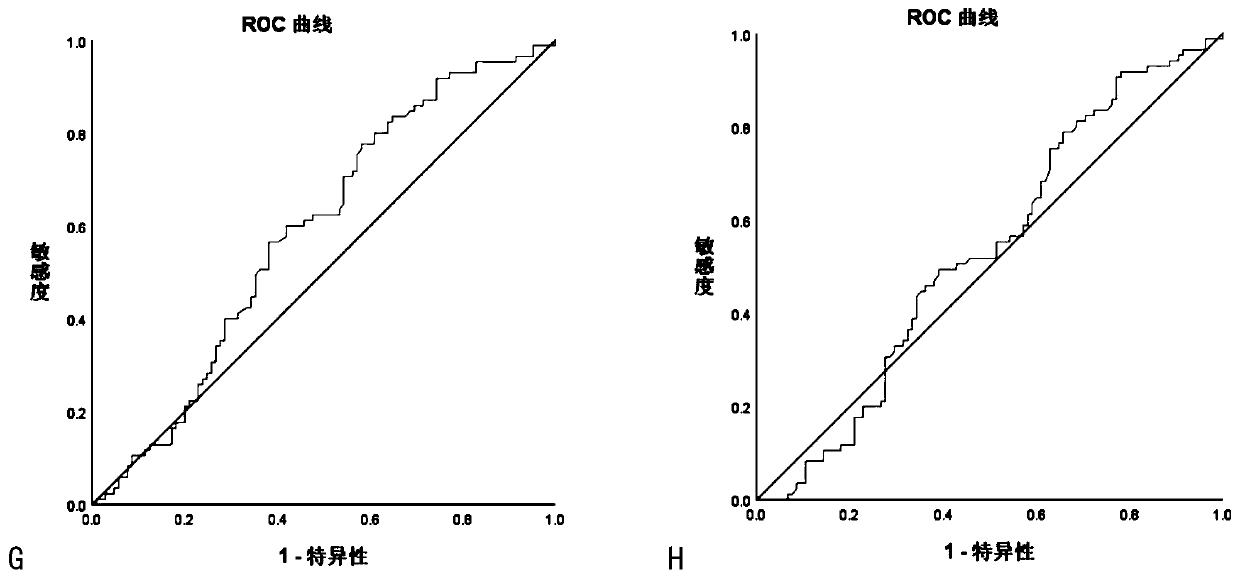
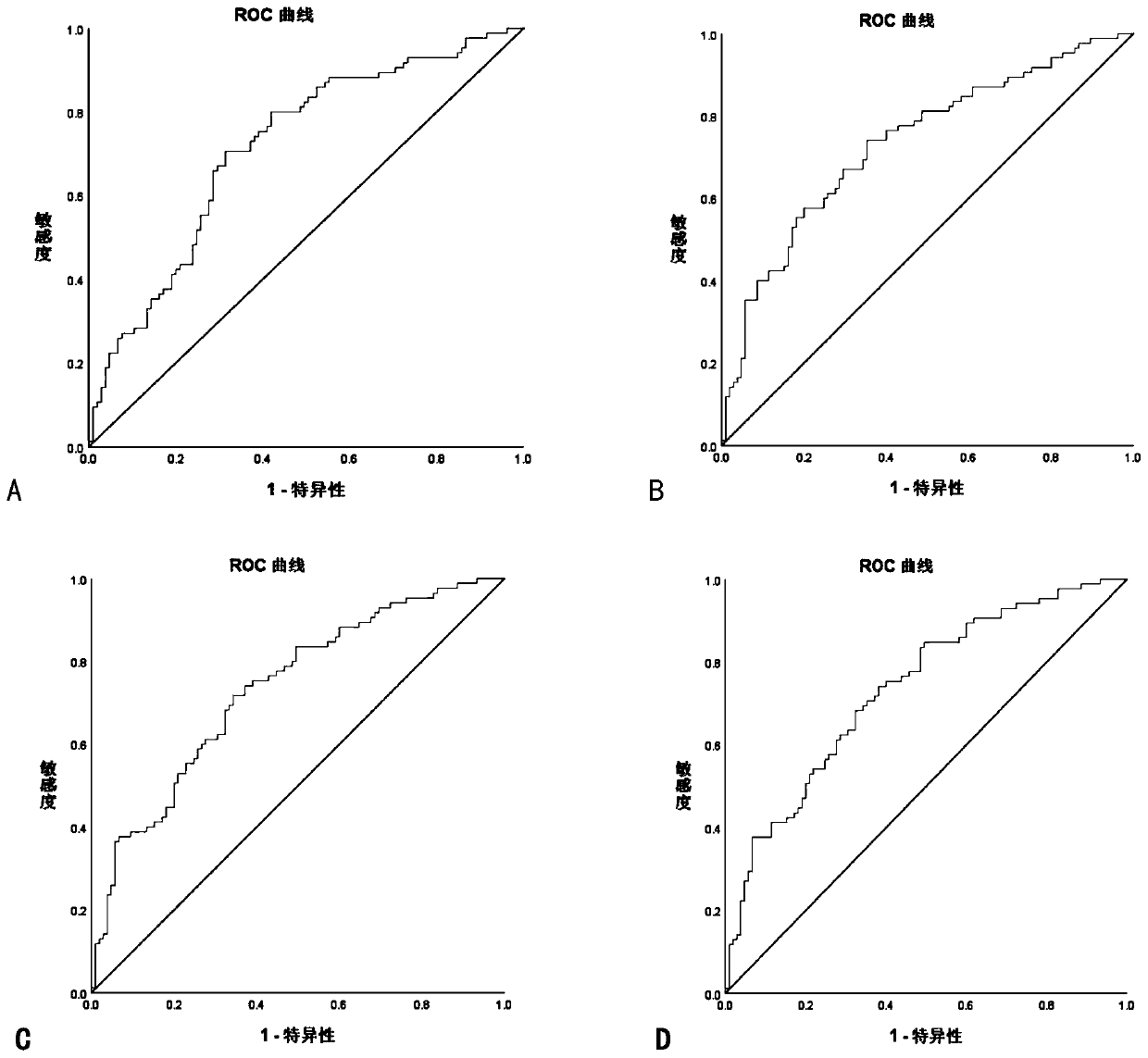
Image
Examples
Embodiment 1
[0085] Example 1. Tumor-associated autoantibodies corresponding to antigenic proteins and anti-tumor marker antibodies were coated with magnetic particles
[0086] The method of directly immobilizing antigenic proteins to magnetic particles is as follows: the recombinant tumor-associated autoantibodies corresponding to antigenic proteins P53, SOX2, CK8, HuD, CAGE, NY-ESO-1, GBU4- 5. P62, KRAS and MAGE A4. Add 2.5 μg recombinant protein to 200 μL magnetic particle suspension (0.05M MES, pH 5.0, containing 1.25×10 6 magnetic particles) and incubate for 2 hours at room temperature in the dark. Then 20 μL of 1 mg / mL EDC was added to the mixture and incubated overnight at room temperature in the dark. Wash 4 times with 1 mL of PBS (containing 1% BSA, 0.2% Tween-20 and 0.05% sodium azide, pH 7.4), 500 μL of blocking / preservation buffer (PBS-TBN containing 0.1% BSA, 0.02% Tween-20 and 0.05 % sodium azide, pH 7.4) to resuspend the magnetic particles and store in the dark at 2-8°C. ...
Embodiment 2
[0089] Example 2. Preparation of kits for detecting tumor-associated autoantibodies and tumor markers
[0090] According to the principles of indirect ELISA and double-antibody sandwich method, the present invention prepares a combined detection kit of multiple autoantibodies and tumor markers that can be used for early screening and diagnosis of lung cancer. The principle of indirect enzyme-linked immunoassay is to connect the antigen protein corresponding to the tumor-associated autoantibody to a solid-phase carrier, and the autoantibody to be tested in the sample is combined with it to form a solid-phase antigen-tested autoantibody complex, and then the enzyme-labeled anti-antibody The human IgG antibody combines with the autoantibody in the solid-phase antigen-test autoantibody complex to form a solid-phase antigen-test autoantibody-enzyme-labeled anti-human IgG antibody complex, and then measure the luminous intensity after adding a luminescent substance to determine Auto...
Embodiment 3
[0108] Embodiment 3, detection method
[0109] 1. Sample and Standard Preparation
[0110] Dilute humanized Anti-His antibody standard 1 twice to obtain standard 2, and then serially dilute to obtain standard 3-6;
[0111] Dilute CEA standard 1, NSE standard 1, and SCC standard 1 by 5 times to obtain CEA standard 2, NSE standard 2, and SCC standard 2, and then serially dilute to obtain CEA standard 3-6, NSE standard 3 ~6 and SCC standard 3~6;
[0112] Part of the serum was diluted 100 times with sample diluent for detection of autoantibodies.
[0113] 2. Detection of autoantibodies
[0114] 2.1 Make a standard curve: draw a standard curve with the luminous intensity as the Y axis and the logarithm of the antibody concentration as the base 10 as the X axis, and calculate the standard curve regression equation.
[0115] 2.1.1 Add 50 μL of standard substance 1-6 into 12 reaction cups respectively, and divide into 6 groups;
[0116] 2.1.2 Add 20 μL Vector-His magnetic particl...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap