Improved methods of assessing uch-l1 status in patient samples
A UCH-L1, state-of-the-art technology, applied in chemical instruments and methods, biochemical equipment and methods, instruments, etc., can solve problems such as lack of standardization of assays, chronic neurodegenerative diseases, neuronal dysfunction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
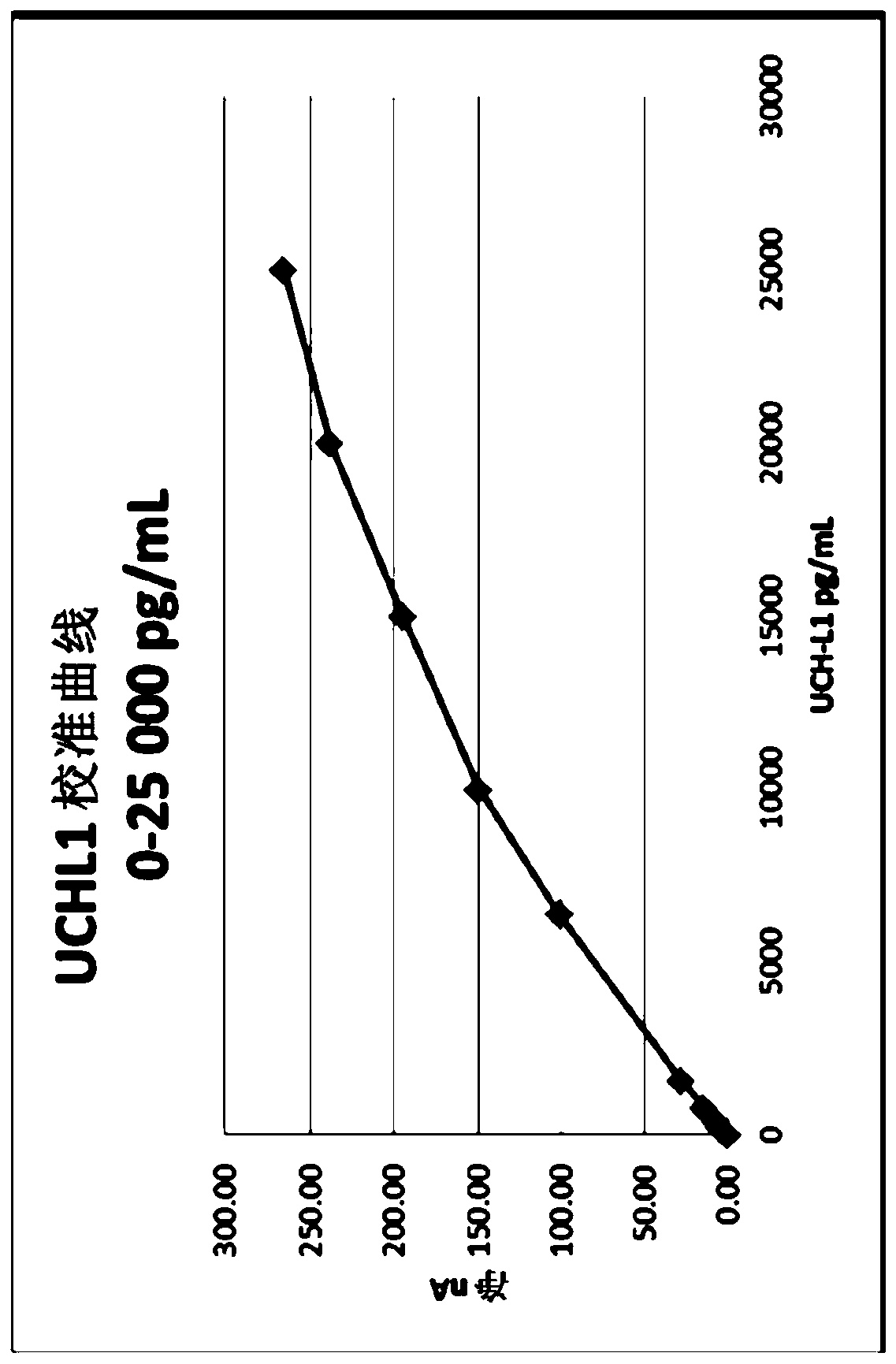
[0389] UCH-L1 assay
[0390] Antibodies were screened using an assay format of interest (i-STAT). Pairs of antibodies that produced a signal in the assay were selected. Initial selection conditions were based on a number of factors, including detection of signal by antibody pairs when screening with low calibrator concentrations. Pairs of monoclonal antibodies were tested, eg Antibody A as capture mAb, and Antibodies B and C as detection mAbs. Antibody A is an exemplary anti-UCH-L1 antibody developed in-house at Abbott Laboratories (Abbott Park, IL). Antibody B and Antibody C are exemplary anti-UCH-L1 antibodies developed at Banyan Biomarkers, Inc. Antibodies B and C recognize different epitopes of UCH-L1 and can enhance the detection of antigens in samples. When used together, the combination of these antibodies provides a synergistic effect and may enhance signaling. Other antibodies developed in-house at Abbott Laboratories (Abbott Park, IL) have shown or are predict...
Embodiment 2
[0422] Possible Assay Interference
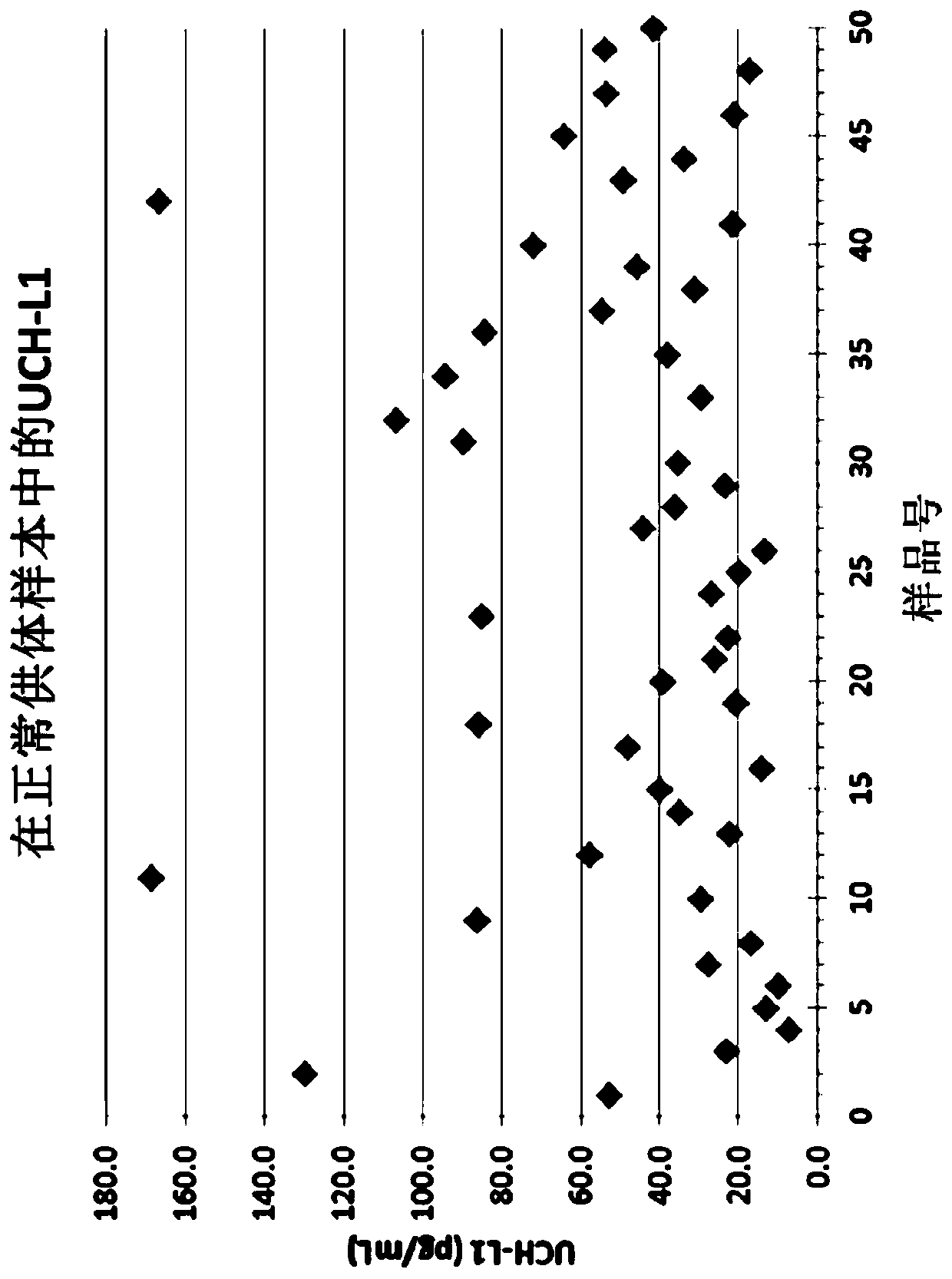
[0423] The UCH-L1 assay was evaluated for possible interferences and cross-reactants, as shown in Table 11. In summary, interferors were spiked into the UCH-L1 panel at target concentrations of 200-250 pg / mL. The test concentration of interfering substances is based on CLSI EP7-A2 guidance (Clinical and Laboratory Standards Institute. Interference Testing in Clinical Chemistry; Approved Guideline-Second Edition. CLSI document EP7-A2 [ISBN 1-56238-584-4]. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2005.). Possible cross-reactants were tested at 500 ng / mL. The acceptance criterion was <10% interferents.
[0424] Specifically, the UCH-L1 assay was evaluated for possible endogenous interference. Potential interfering substances were prepared in preferred buffers / solvents and added to test samples containing the analytes of interest. Control samples were prepared where o...
Embodiment 3
[0435] TBI Population Study
[0436] The i-STAT UCH-L1 assay was used in a TBI patient population study.
[0437] Study Samples: A total of 260 subjects with moderate to severe TBI were enrolled, up to 8 time points / subject; all samples were serum. Table 12; FIG3.
[0438] Table 12
[0439]
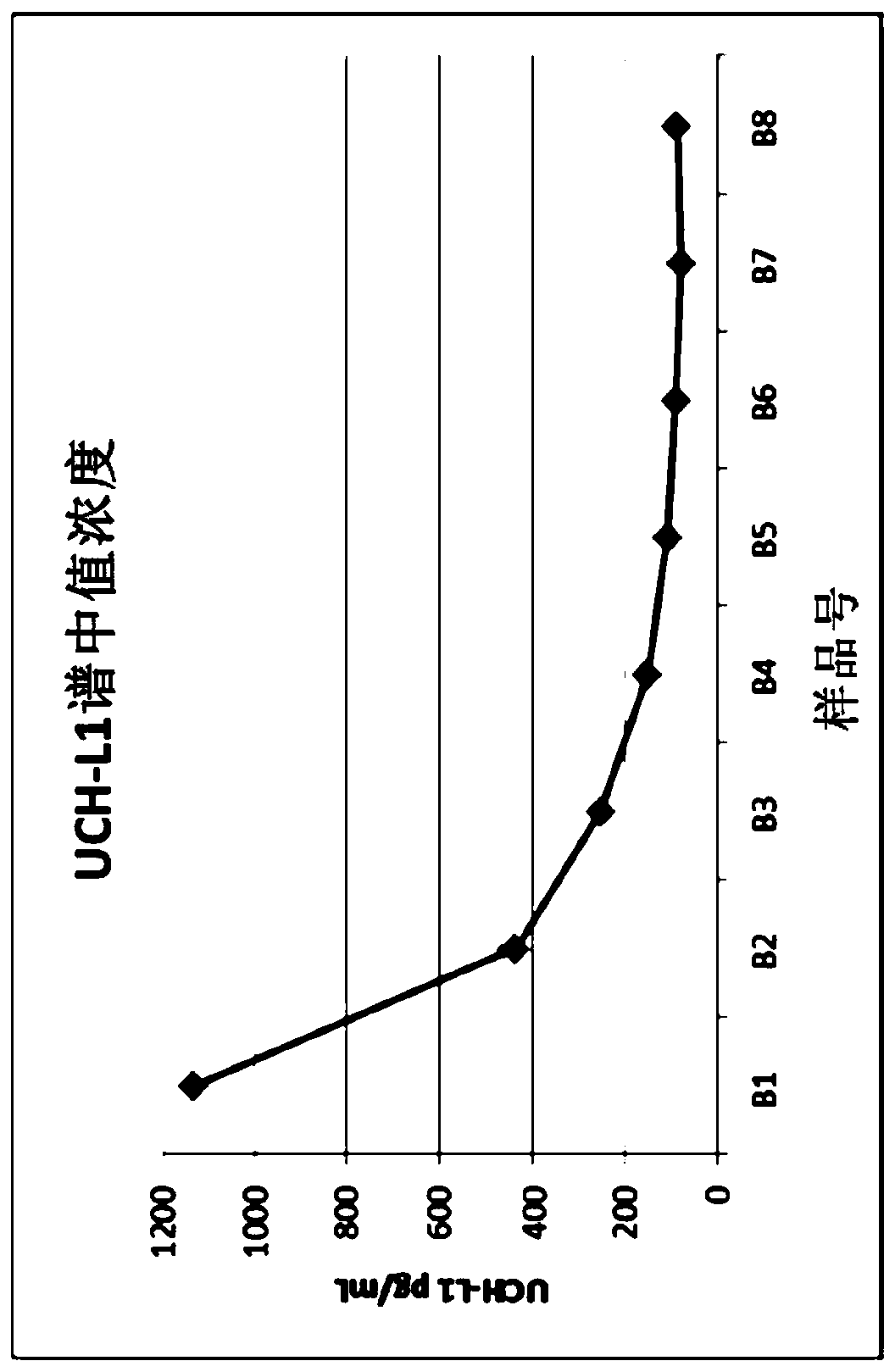
[0440] Assignment of study samples. image 3 Median results of all UCH-L1 assay results at each time point are shown, indicating that UCH-L1 is higher on B1 samples and tends to decrease with later time points. Figure 4 Shown are boxplots (log scale) at each sample time point showing broad distribution of UCH-L1 results in the patient population. The boxes represent interquartile ranges (25th, 50th and 75th percentiles).
[0441] In all, a total of 250 subjects were available for testing. Not all time points are necessarily available from all subjects. Some samples had limited or insufficient volume. UCH-L1 results ranged from <0.05 to 12,100 pg / mL, and these results were read...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com