Mutant of D-allulose 3-epimerase and application of mutant
An epimerase and psicose technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of poor temperature stability, unsuitable for industrial production, low fructose catalytic activity, etc., and achieve conversion rate improvement and temperature stability. The effect of improving and improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
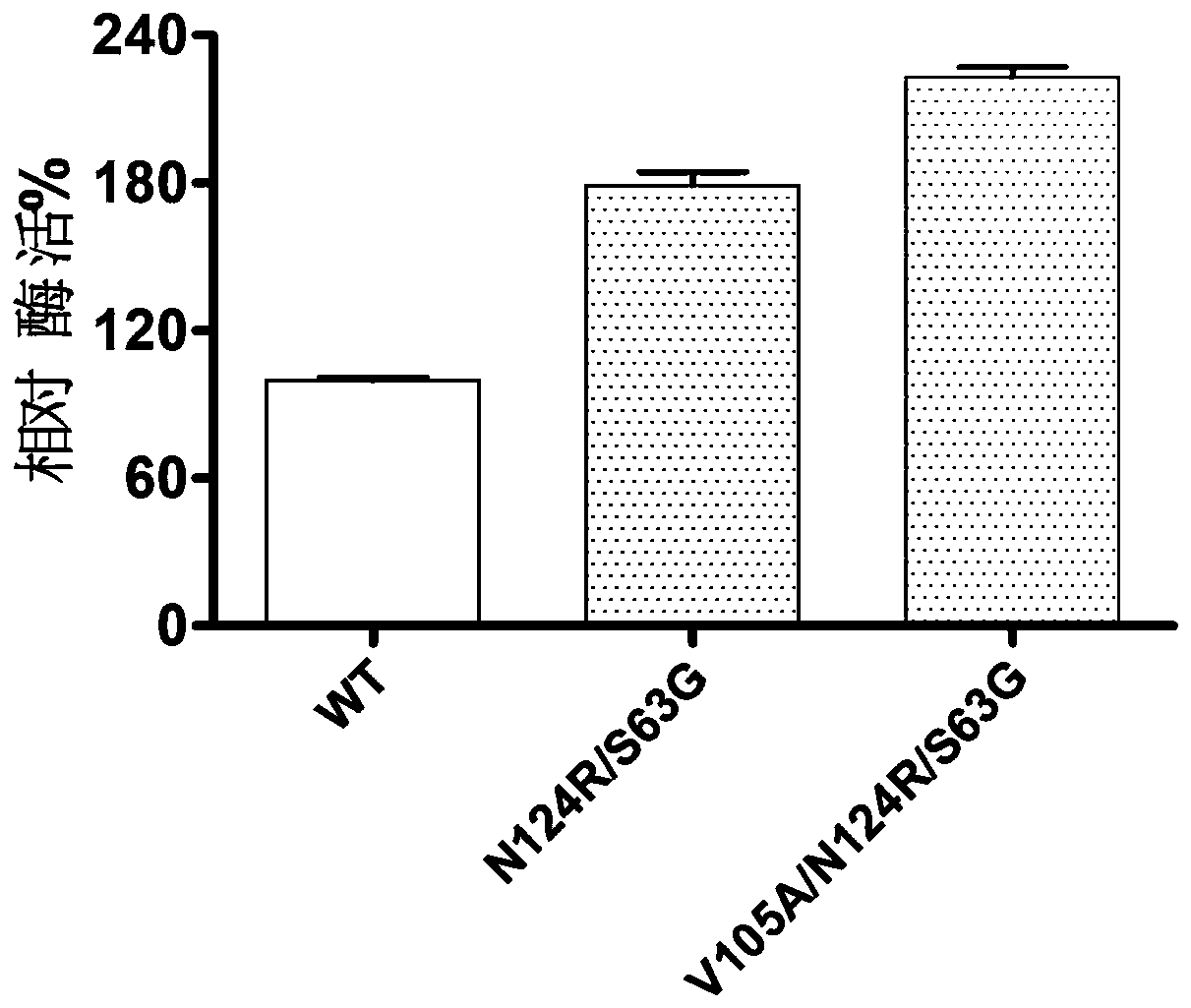
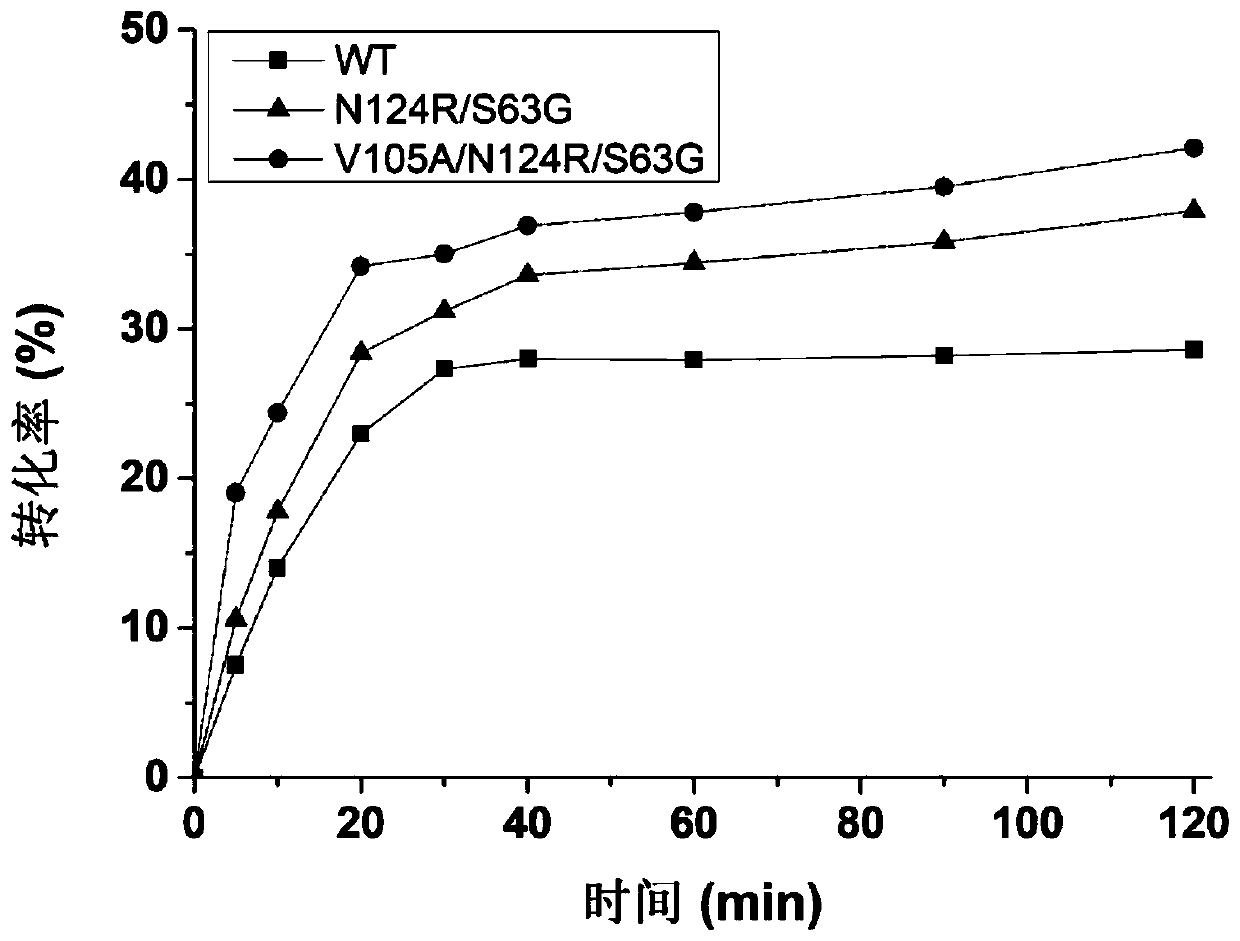
[0034] Example 1: Construction of D-psicose-3-epimerase mutants
[0035] (1) AgDAE-pET-28a plasmid (Suzhou Jin Weizhi Biotechnology Co., Ltd.) was used as a template, and random mutations were performed using the GeneMorph II random mutation kit (Cat. No.: 200550) from Agilent Technologies.
[0036] (2) Amplify the AgDAE gene by the following primers
[0037] Primer (5'-3')AgDAE-F:
[0038] GGAATTCCATATGAAAATTGGCTGTCATGGTCTG,
[0039] Primer (5'-3')AgDAE-R:
[0040] CCGCTCGAGT TAATGCAGCT CGATGGTCTT GATTG,
[0041] The AgDAE gene fragment of about 870bp was amplified by error-prone PCR technique.
[0042] Error-prone PCR reaction system (50μL):
[0043] Add the reagents to the 0.2mL EP tube in the following order:
[0044]
[0045] PCR reaction conditions:
[0046]
[0047] After the PCR reaction, 2 μL of the amplified product was subjected to agarose gel (0.8%) electrophoresis, the result was observed, and the PCR product at about 870 bp was purified and recovered....
Embodiment 2
[0074] Example 2: Activation and induced expression of mutants
[0075] Pick a single clone from the plate cultured overnight to a 96-deep well plate, fill each empty plate with 200 μL of LB medium containing 50 μg / mL kana antibiotics, cultivate overnight at 37°C, and then pipette 10 μL of the overnight cultured bacterial solution to the plate containing 800 μL 96-deep-well plate of LB medium containing 50 μg / mL kanabioxin, cultured at 37°C for about 2 hours, until OD 600 Add IPTG with a final concentration of 0.5mM between 0.6-0.8, and culture at 16°C for 16-20h. Centrifuge at 5000r / min for 30min to collect the bacteria, resuspend in LysisBuffer (20mM Tirs-HCl 20mM imidazole 500mM NaCl pH=7.4), mix well and add 200μL lysozyme, 300μL PMSF and 0.1% (v / v) TritonX-100, Incubate at 37°C for about 2 hours. Centrifuge at 5000r / min for 30min, draw 100 microliters of supernatant into a new 96-well plate, add 1% (w / v) fructose, react at 60°C for 10min, boil the reaction solution for ...
Embodiment 3
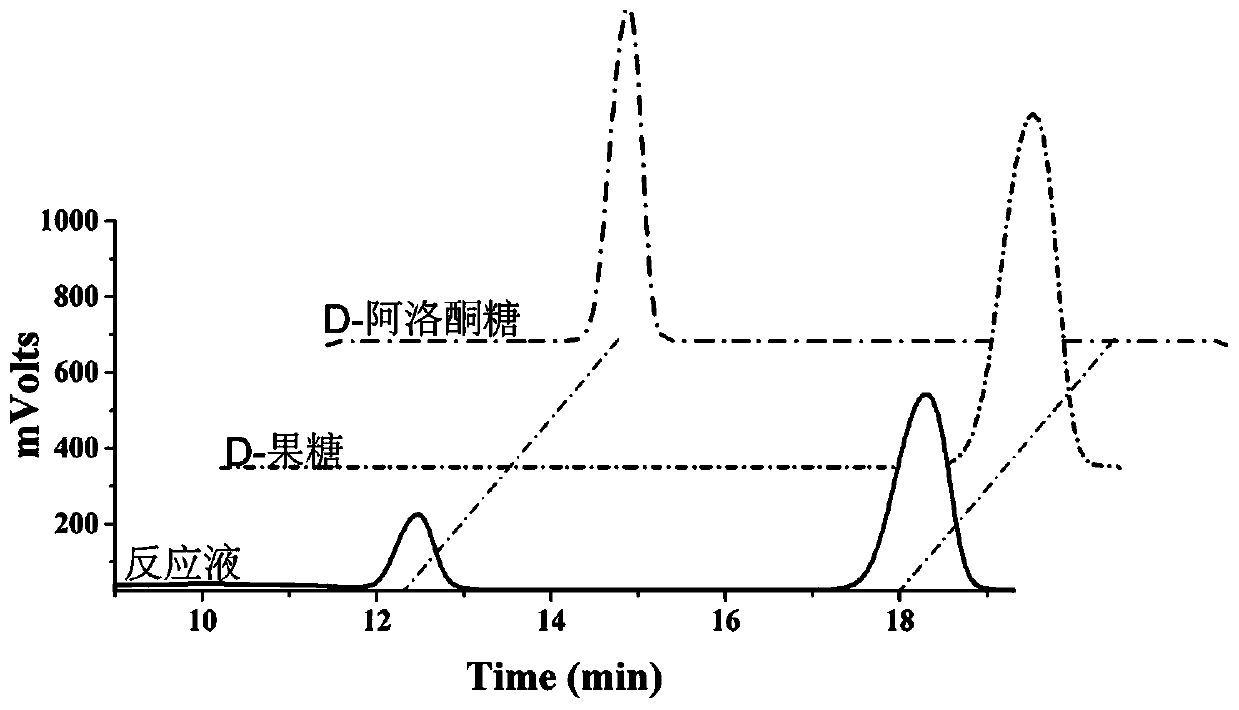
[0076] Embodiment 3: HPLC identifies the generation of D-psicose product
[0077] HPLC detection conditions are:
[0078] Chromatograph: Agilent1260;
[0079] Detector: Evaporative Light Scattering Detector (Alltech Chrom, ELSD6000)
[0080] Injection: Agilent autosampler; injection volume 20 μL;
[0081] Chromatographic column: Prevail Carbohydrate ES column-W (5μm, 4.6×250mm, Agela Technologies, China); column temperature 40°C;
[0082] Mobile phase: 85% acetonitrile; flow rate 1 mL / min.
[0083] The result is as figure 1 As shown, the substrate D-fructose and the product D-psicose are well retained and separated in the column.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap