Interleukin-2 immunoconjugate, cd40 agonist, and optionally pd-1 axis binding antagonist for use in methods of treating cancer
An immunoconjugate, PD-1 technology, applied in the field of interleukin-2 immunoconjugates, CD40 agonists, and optionally PD-1 axis binding antagonists for use in the treatment of cancer, can address advanced cancer patients with poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The term "pharmaceutical composition" refers to a preparation which is in a form which permits the biological activity of the active ingredients to be effective and which is free of additional ingredients which are unacceptably toxic to a subject to whom the formulation will be administered. Preferably, such compositions are sterile.
[0080] "Pharmaceutically acceptable carrier" refers to ingredients other than the active ingredient in the pharmaceutical composition that are non-toxic to the subject. Pharmaceutically acceptable carriers include, but are not limited to, buffers, excipients, stabilizers or preservatives.
[0081] As used herein, the term "treatment / treatment" refers to a clinical intervention designed to alter the natural course of a treated / treated individual or cell during the course of a clinical pathology. Desired effects of treatment / treatment include decreased rate of disease progression, amelioration or palliation of disease state, and regression...
Embodiment 1
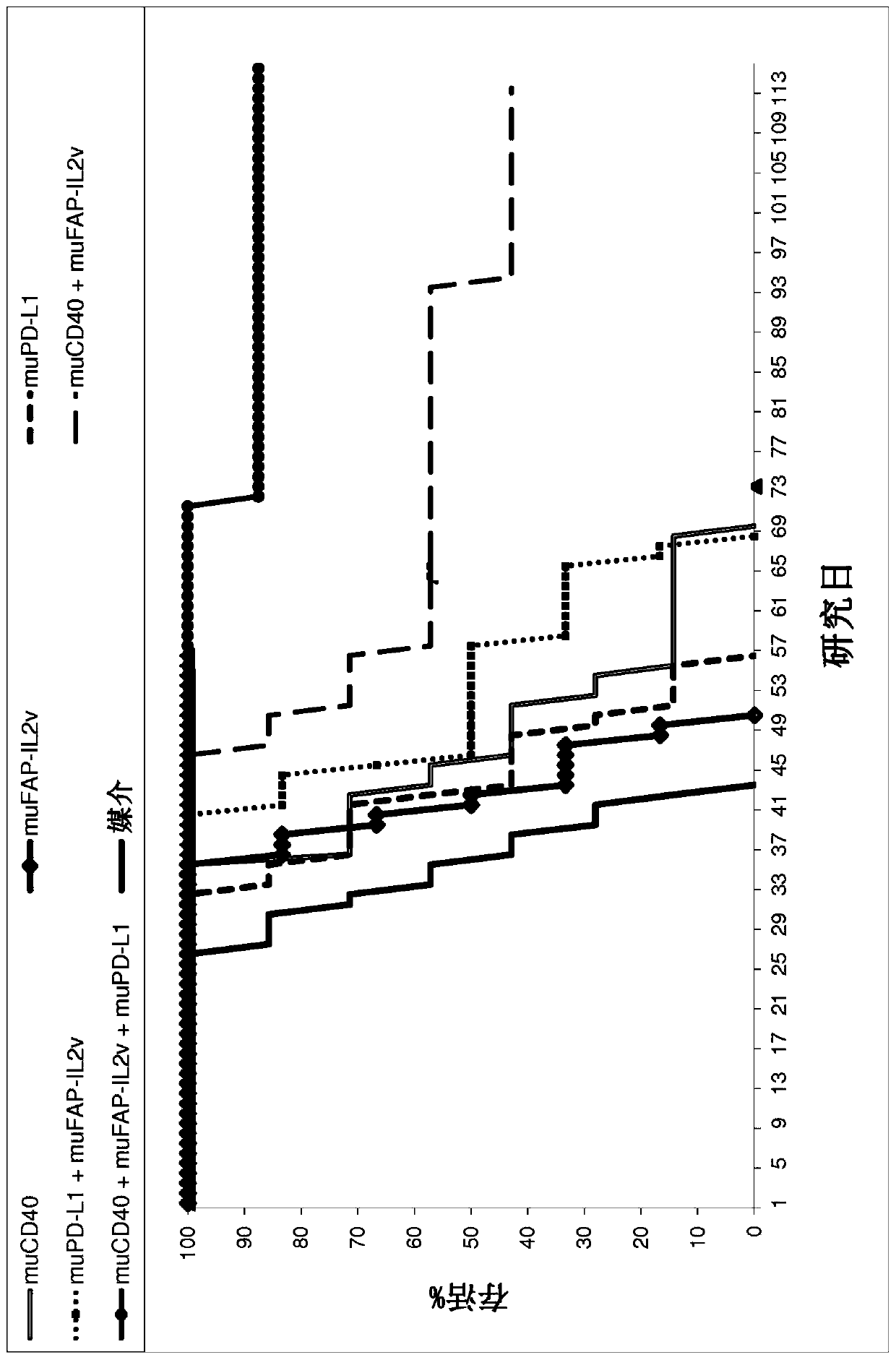
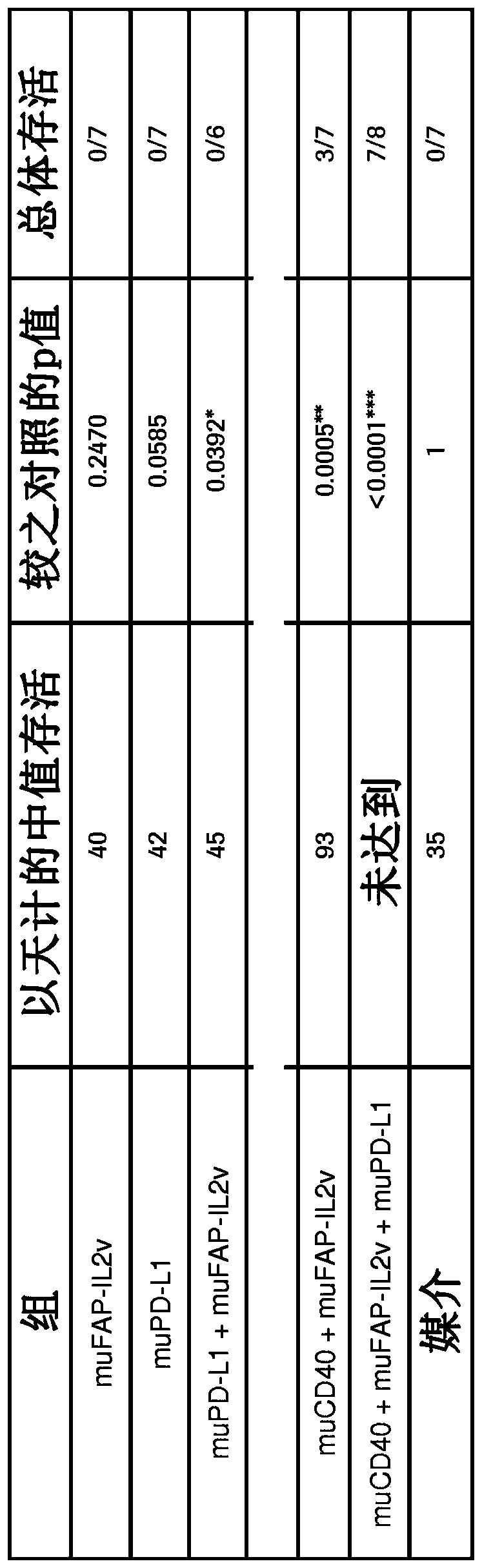
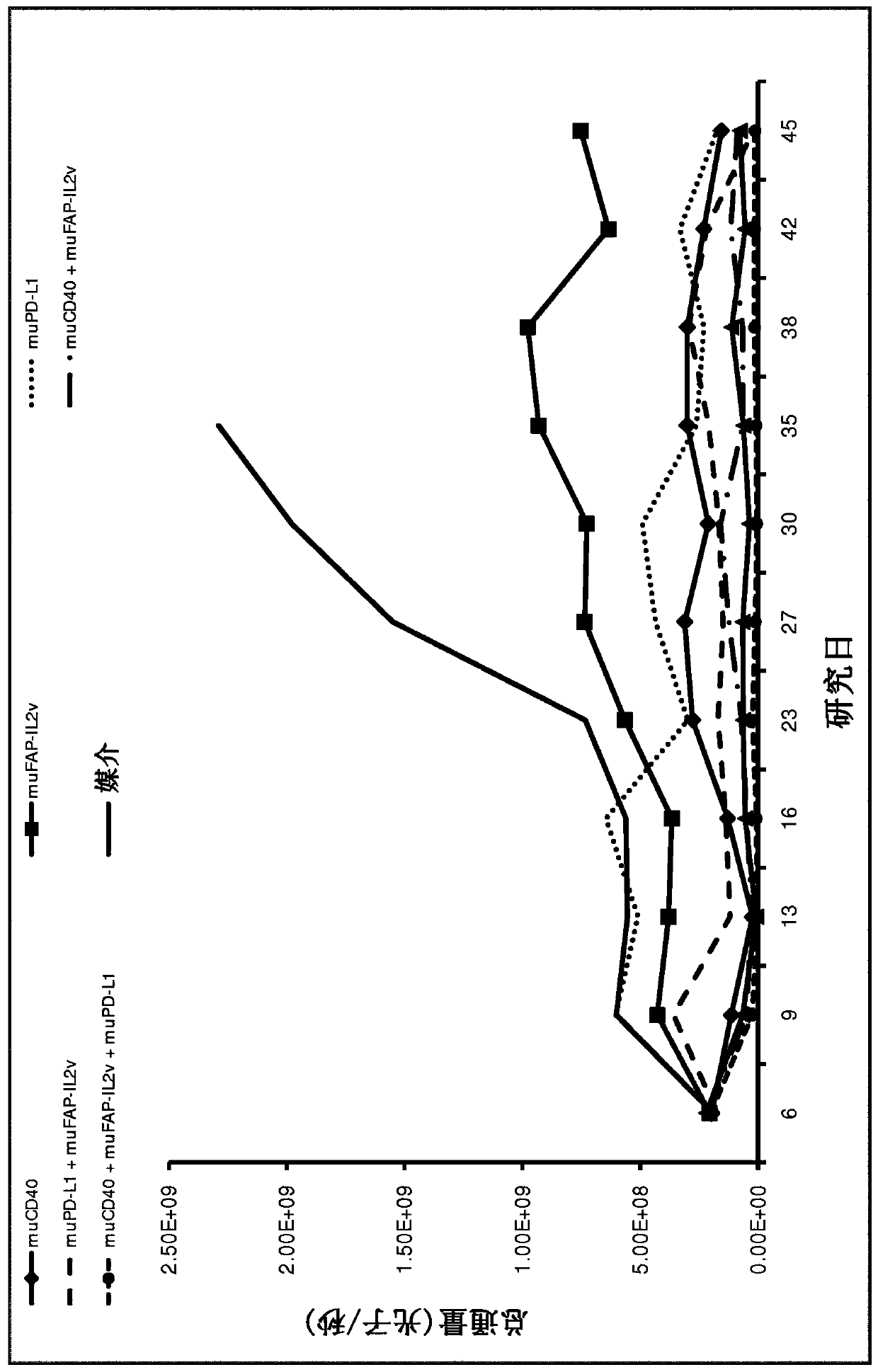
[0444] Example 1: In vivo efficacy of FAP-targeted IL2v immunoconjugates alone and in combination with anti-CD40 mAbs and anti-PD-L1 mAbs in a mouse tumor cell line syngeneic model
[0445] FAP-targeted IL2v immunoconjugates alone and in combination with CD40 mAb and PD-L1 mAb were tested for their antitumor efficacy in a syngeneic mouse model.
[0446] Panc02-Fluc isogenic model of pancreas
[0447] The murine surrogate FAP-targeting FAP-IL2v immunoconjugate was tested in the mouse pancreatic Panc02-Fluc transfectant cell line injected intrapancreatically into Black 6 mice.
[0448] Panc02-H7 cells (pancreatic carcinoma in mice) were originally obtained from MD Anderson Cancer Center (Texas, USA) and deposited at the Roche-Glycart internal cell bank after expansion. The Panc02-H7-Fluc cell line was generated in-house by calcium transfection and subcloning techniques. Panc02-H7-Fluc was cultured in RPMI medium containing 10% FCS (Sigma), 500 μg / ml hygromycin and 1% Glutama...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com