Immunoconjugates comprising mutant interleukin-2 and an anti-CD8 antibody
A technology of immunoconjugates and interleukins, applied in the field of immunoconjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] The term "pharmaceutical composition" refers to a preparation which is in a form which allows the biological activity of the active ingredients contained in the preparation to be effective and which does not contain a or additional components with unacceptable toxicity.
[0101] "Pharmaceutically acceptable carrier" refers to non-toxic components in a pharmaceutical composition other than the active component to the subject. Pharmaceutical carriers include, but are not limited to, buffers, excipients, stabilizers, or preservatives.
[0102] As used herein, "treatment" (and its grammatical variants, such as "treat" or "treating") refers to an attempt to alter the natural course of the disease in the individual being treated, and may be performed to treat Clinical interventions for prevention or performed during clinical pathology. Desired effects of treatment include, but are not limited to, prevention of occurrence or recurrence of disease, alleviation of symptoms, at...
example
[0294] The following are examples of methods and compositions of the invention. It is understood that various other embodiments may be practiced, given the general description provided above.
example 11
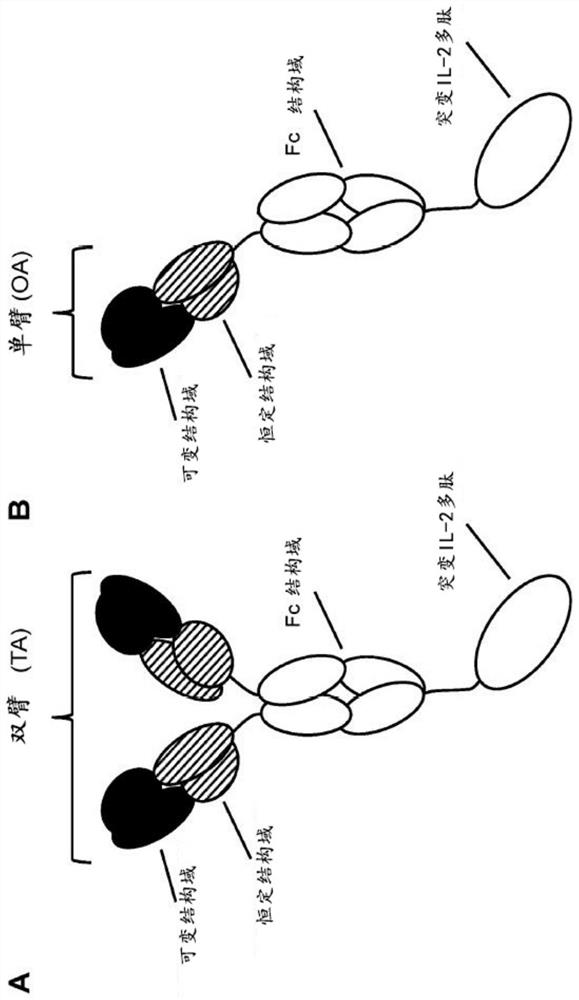
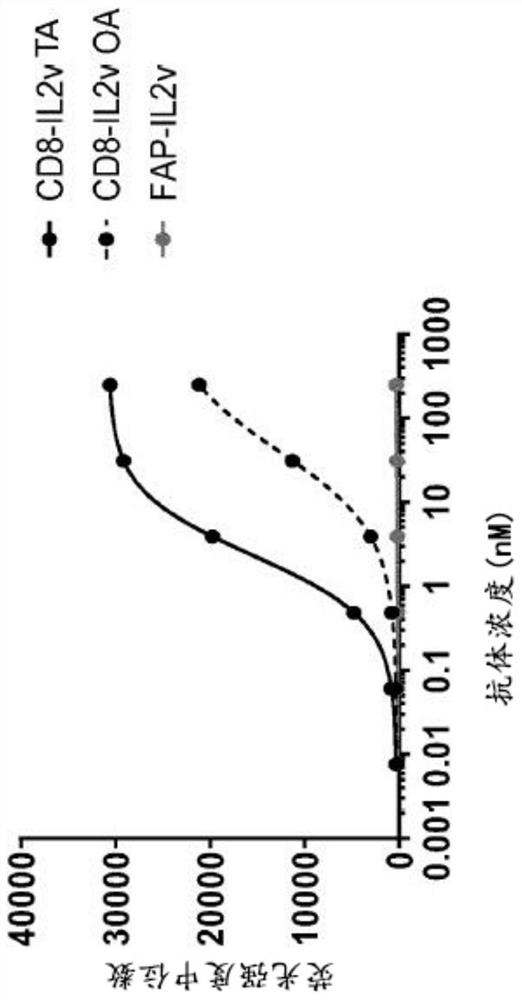
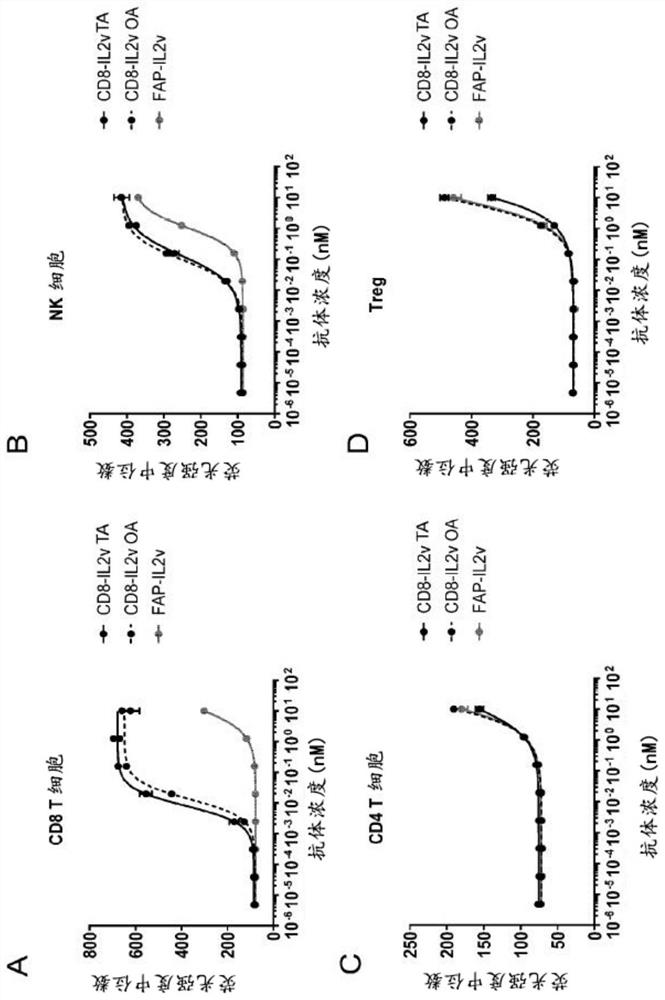
[0296] Example 1.1 Production of anti-CD8-IL2v TA conjugates and anti-CD8-IL2v OA conjugates targeting human CD8
[0297] The abbreviation "TA" means "double arm" herein. The abbreviation "OA" means "single arm" herein. The terms "anti-CD8-IL2vTA" and "CD8-IL2v TA" are used interchangeably herein. The terms "anti-CD8-IL2v OA" and "CD8-IL2v OA" are used interchangeably herein.
[0298] Expression of all genes is under the control of the human CMV promoter-intron A-5'UTR cassette. The BGH polyadenylation signal is located downstream of the gene. For use in HEK293 EBNA cells, the vector contains an oriP element for stable episomal maintenance of the plasmid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com