Pigment Yellow 139 or its derivatives wrapped in thermoplastic resin, its preparation method and its downstream products
A technology of thermoplastic resin, pigment yellow, applied in the field of pigments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the thermoplastic resin-wrapped pigment yellow 139 or its derivatives of one embodiment, comprises the following steps:
[0032] Mix 1,3-diiminoisoindoline compounds, cyclic compounds containing meta-diketones, solid organic acids and thermoplastic resins, and extrude or knead to obtain Pigment Yellow 139 or its derivatives wrapped in thermoplastic resins things.
[0033] Wherein, the structure of 1,3-diiminoisoindoline compounds is shown in formula (I):
[0034] R1, R2, R3, and R4 are each independently H, halogen, C1-C4 alkyl or alkoxy.
[0035] The structure of the cyclic compound containing meta-diketone is shown in formula (II):
[0036] X are independently CH 2 Or NH, Y is C=O, S, CR5R6 or NR7, R5, R6, R7 are independently H, methyl, ethyl or benzyl.
[0037] Further, the cyclic compound containing meta-diketone is selected from one of the following compounds:
[0038] The definitions of R5, R6 and R7 are the same as above.
...
Embodiment 1
[0071]
[0072] Mix (20g, 138mmol) 1,3-diiminoisoindoline, (35g, 276mmol) barbituric acid, (6.6g, 34mmol) citric acid and 0.5kg of PMMA in a single-screw extruder Extrusion reaction, pelletizing, to obtain crude color masterbatch.
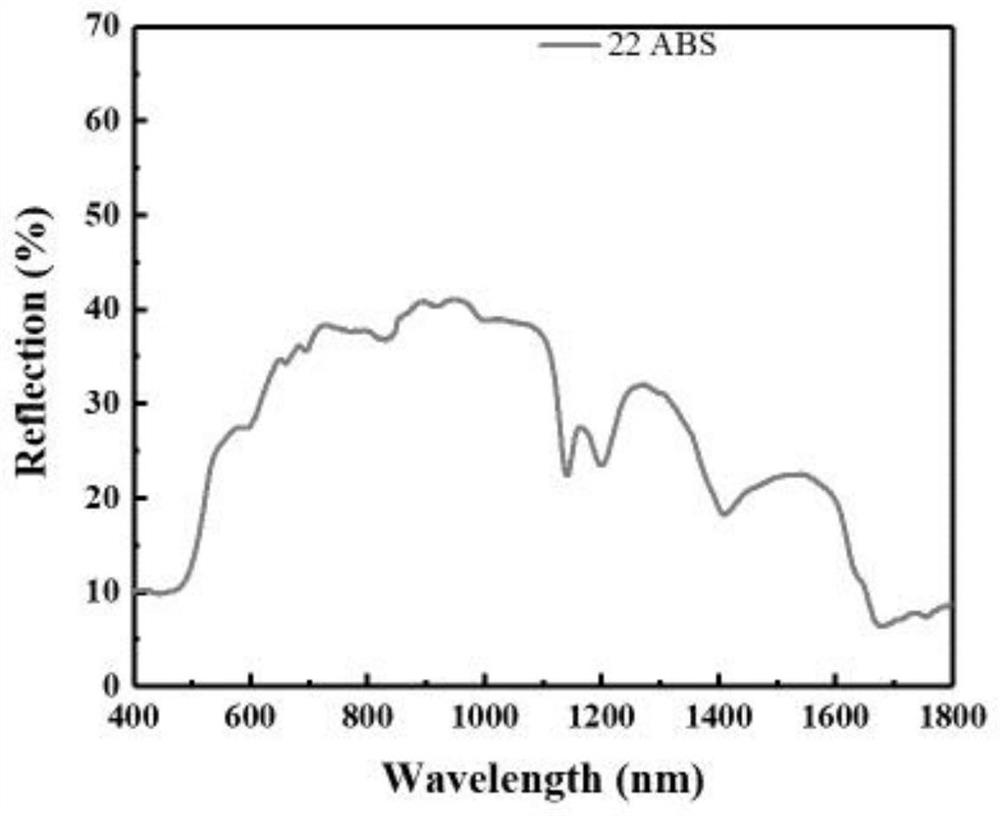
[0073] figure 1 The reflectance spectrogram of injection molding into sheet after mixing ABS for the color masterbatch crude product prepared in embodiment 1, by figure 1 It was found that the obtained plastic sheet was yellow.
Embodiment 2
[0075] Mix (20g, 138mmol) 1,3-diiminoisoindoline, (35g, 276mmol) barbituric acid, (6.6g, 34mmol) citric acid and 0.5kg of PP in a single-screw extruder Extrusion reaction, pelletizing, to obtain crude color masterbatch.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com