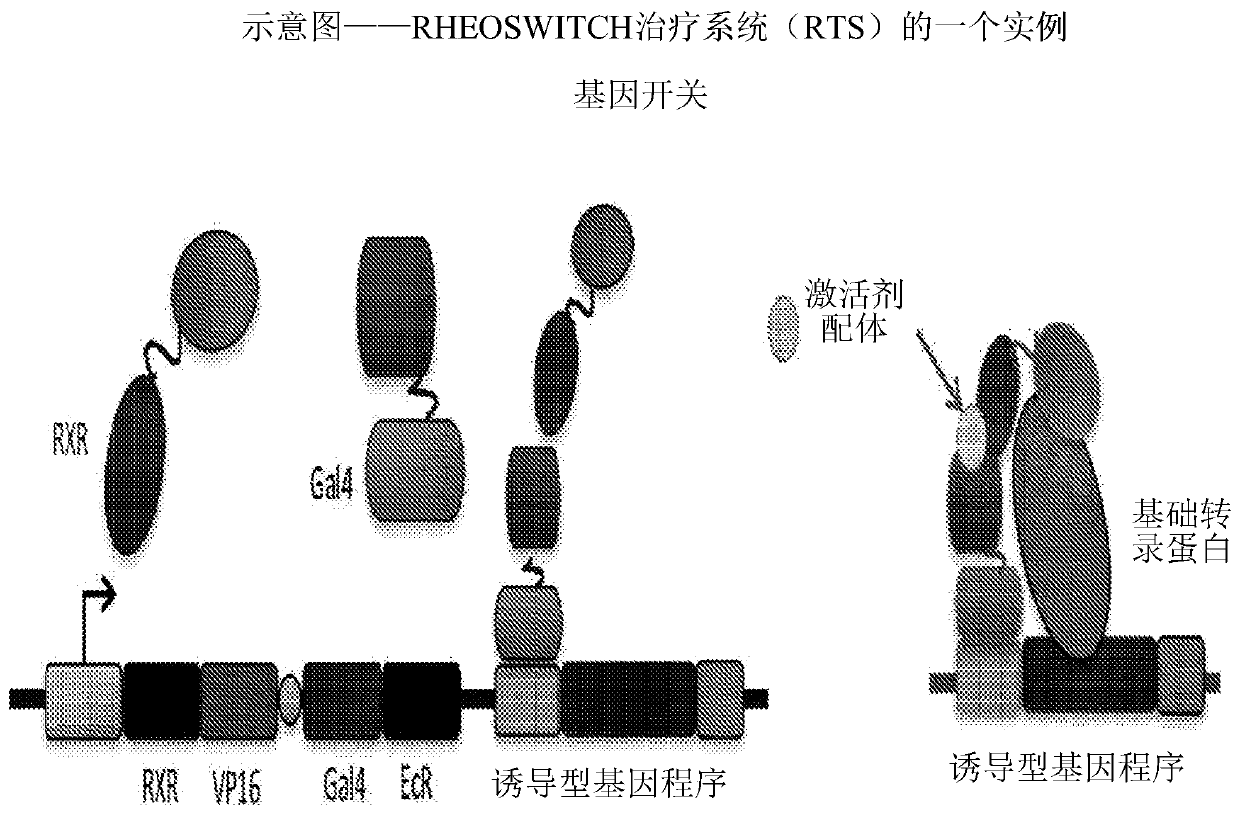
Delivery of autologous cells comprising matrix metalloproteinase for treatment of scleroderma
A matrix metal and protease technology, applied in the direction of medical preparations containing active ingredients, animal cells, skin diseases, etc., can solve the problem of heavy treatment work, long-term prevention of long-term active disease or recurrence, dosage regimen or frequency and total Issues such as lack of consensus on exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
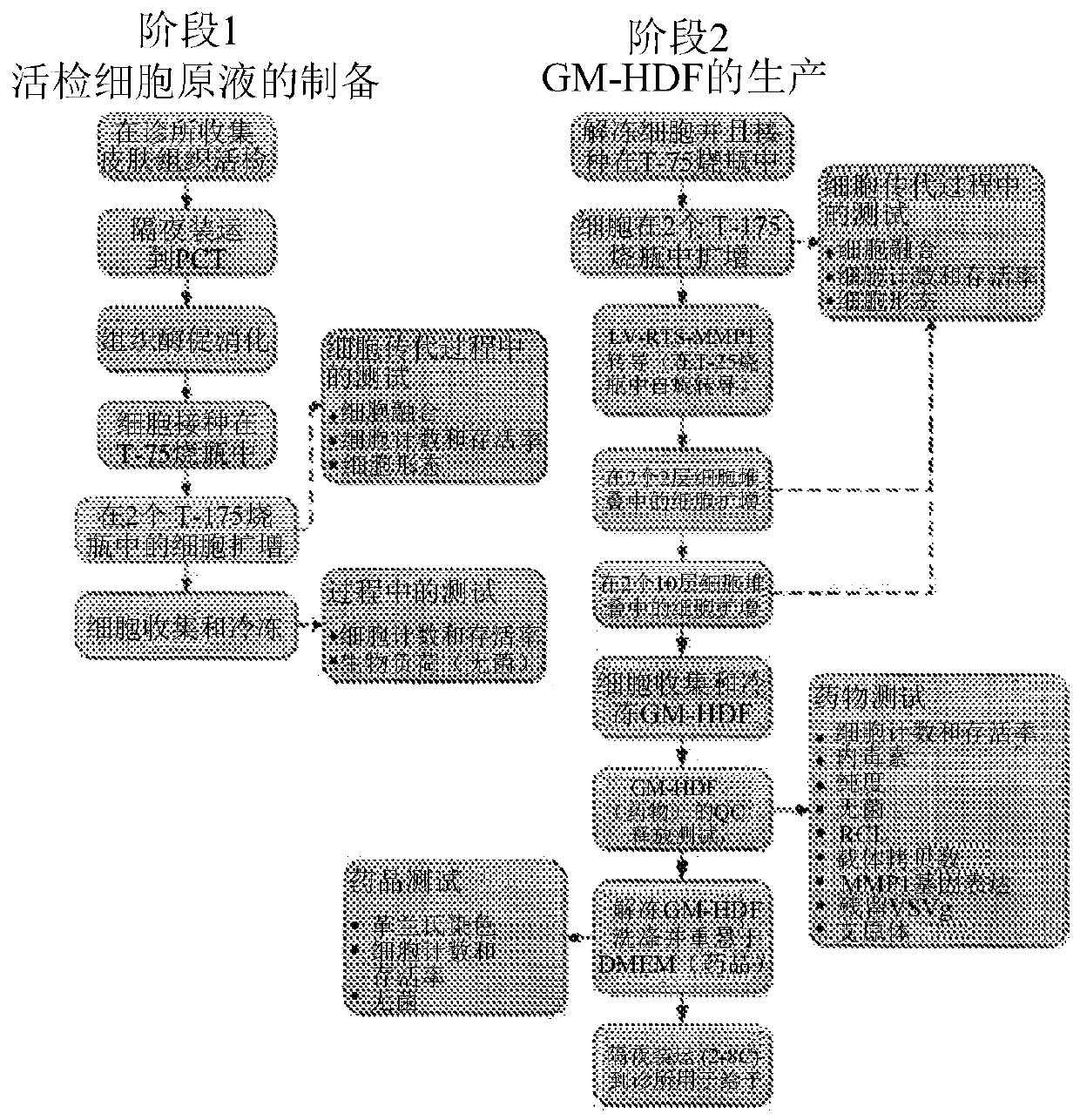
Image
Examples
example 1
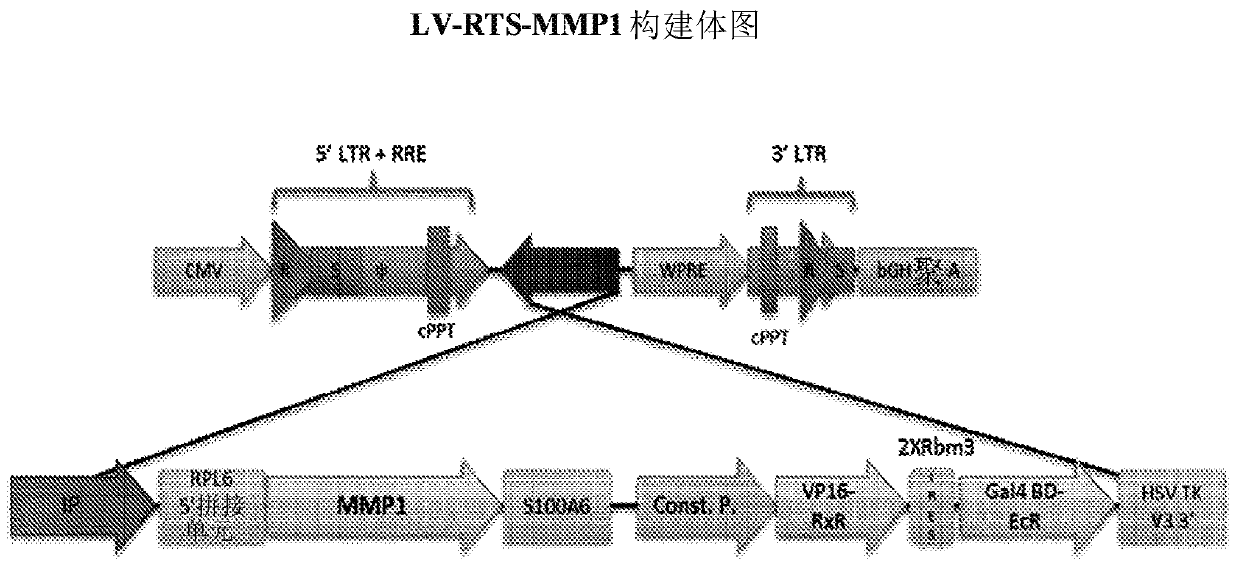
[0122] Construction and characterization of LV-RTS-MMP1 lentivirus
[0123] The recombinant lentiviral vector (LV) LV-RTS-MMP1 was used to introduce the MMP1 gene into cultured fibroblasts. The LV is a non-replicating vesicular stomatitis virus-G (VSV-G) pseudotyped, self-inactivating (3rd passage) lentivirus (SIN-LV). LV-RTS-MMP1 has a nucleotide sequence corresponding to SEQ ID NO:1, and an amino acid sequence has a sequence of SEQ ID NO:3.
[0124] Source of human MMP1 gene
[0125]The sequence of MMP1 cDNA (SEQ ID NO:1) was derived from the consensus sequence of human pro-MMP1, wherein the native MMP1 signal peptide was replaced by the signal peptide sequence (SEQ ID NO:2) of human pigment epithelium-derived factor (PEDF) to provide More efficient secretion of MMP1 from fibroblasts. The cDNA (1410 bp) was generated and cloned into standard expression plasmids for initial analysis. The MMP1 cDNA was then engineered to remove potential splice sites and cloned into th...
example 2
[0126] Example 2: FCX-013 and Veledimex background
[0127] 2.1 In vitro studies
[0128] Veledimex-induced expression of MMP1 was assessed in primary fibroblasts genetically modified by transduction with LV-RTS-MMP1. Primary normal human dermal fibroblasts (NHDF) were transduced with different dilutions of research grade LV-RTS-MMP1 stock solution. Two passages after transduction, transduced NHDFs (HDF-RTS-MMP1 ) were seeded into 24-well plates and treated with 100 nM veledimex or 0.1% DMSO (vehicle). The study also included NHDF not transduced with LV-RTS-MMP1 ("mock"). Cell supernatants were collected 72 hours after addition of veledimex or DMSO and analyzed for MMP1 expression levels by ELISA (R&D Systems DuoSet). Cells were collected and analyzed for integrated LV-RTS-MMP1 copy number (average per cell) by qPCR using primers and a probe specific for the LV-RTS-MMP1 construct. The results of the three transduction studies are detailed in Table 2 below, and in Figur...
example 3
[0145] Example 3: Studies in NOD / SCID mice
[0146] This example describes studies using a bleomycin-induced (BLM-induced) disease model in NOD / SCID mice. Figure 8 An overview of the progress of the treatment is shown.
[0147] Table 5 provides a description of the study groups.
[0148] The design of this study was based on experimental data (data not shown) which showed that:
[0149] In NOD / SCID mice given FCX-013 plus veledimex, MMP1 expression (both protein and mRNA) was maximal between 24 hours and 3 days and was undetectable at 28 days after FCX-013 injection.
[0150] Protein expression was higher in BLM-treated animals compared to non-BLM-treated animals.
[0151] The observed systemic toxicity was attributed to bleomycin treatment.
[0152] No established toxicity with 2x 10 6 FCX-013 cell related
[0153] On day 10 after injection of FCX-013, DNA copy number and MMP1 expression decreased rapidly
[0154] To ensure adequate MMP1 expression, copy number target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com