Lysis solution for magnetic bead method HCMV nucleic acid extraction and application of lysis solution
A technology of lysate and magnetic bead method, which is applied in the field of molecular biology, can solve the problem that the antibody cannot respond to the HCMV load, and achieve the effect of efficient extraction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The preparation method of the lysate for extracting HCMV nucleic acid by the magnetic bead method provided by the invention at least comprises the following steps:
[0048] Mix guanidine isothiocyanate, sodium lauryl sulfate, NP40, Tris-HCL, EDTA and water, adjust the pH value of the lysate, and add β-mercaptoethanol to obtain the lysate.
[0049] Further, the pH value of the lysate is adjusted to 8.5±0.1.
[0050] The invention provides a lysate for HCMV nucleic acid extraction by magnetic bead method for preparing HCMV nucleic acid extraction products in samples or for HCMV nucleic acid extraction in amniotic fluid.
[0051] The HCMV nucleic acid sample can be selected from amniotic fluid samples.
[0052] When extracting HCMV nucleic acid from a sample, the required sample volume is at least 200 μL. Further, it can be 200-250ul.
[0053] A kit for extracting HCMV nucleic acid by magnetic bead method provided by the present invention includes the aforementioned lysat...
Embodiment 1
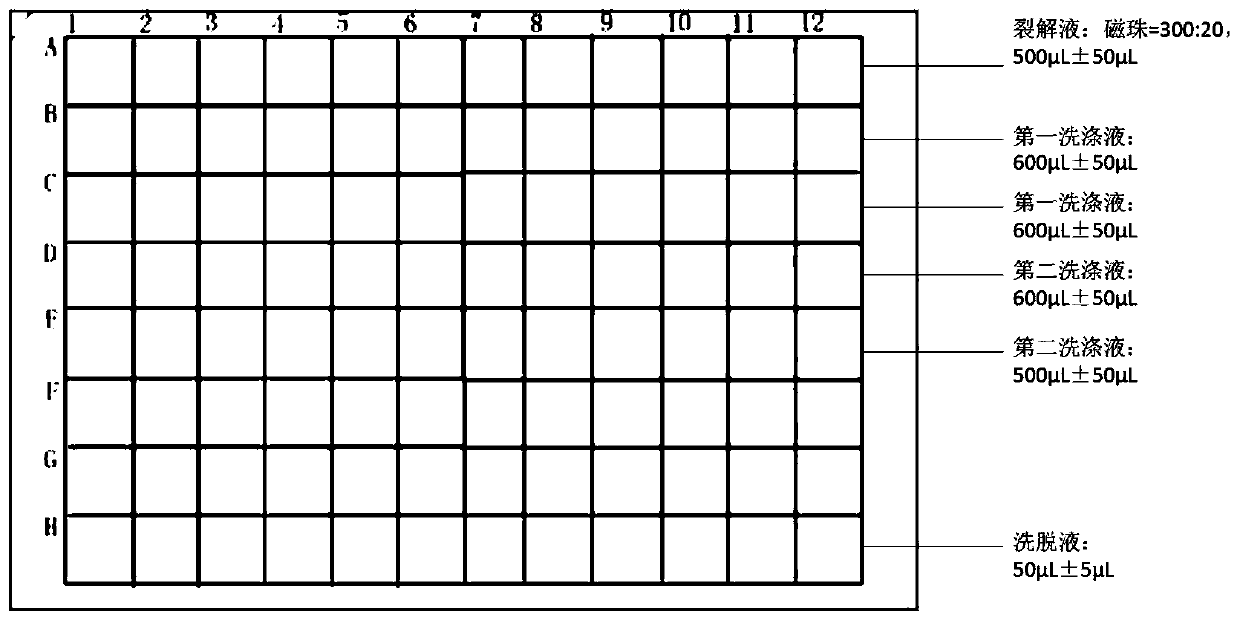
[0078] Prepare the test kit of magnetic bead method HCMV nucleic acid extraction with following formula, described test kit comprises lysate, magnetic bead, the first washing liquid, the second washing liquid, eluent, RNA precipitation aid and proteinase K, wherein
[0079] Based on the total amount of the lysate, the content of each component of the lysate is:
[0080]
[0081]
[0082] Based on the total amount of the first washing liquid, the content of each component of the first washing liquid is: guanidine isothiocyanate: 2mol / L, sodium chloride: 0.5mol / L, triton X-100: 1 % (w / v), absolute ethanol: 50% (v / v), Tris-HCl: 2mmol / L, solvent is water.
[0083] Based on the total amount of the second washing liquid, the content of each component of the second washing liquid is: absolute ethanol: 75% (v / v), and the solvent is water.
[0084] Based on the total amount of the eluent, the content of each component of the eluent is: 40mmol / L, EDTA 10mmol / L, and the solvent is...
Embodiment 2
[0090] 1. Experimental method
[0091] 1.1 Detection object
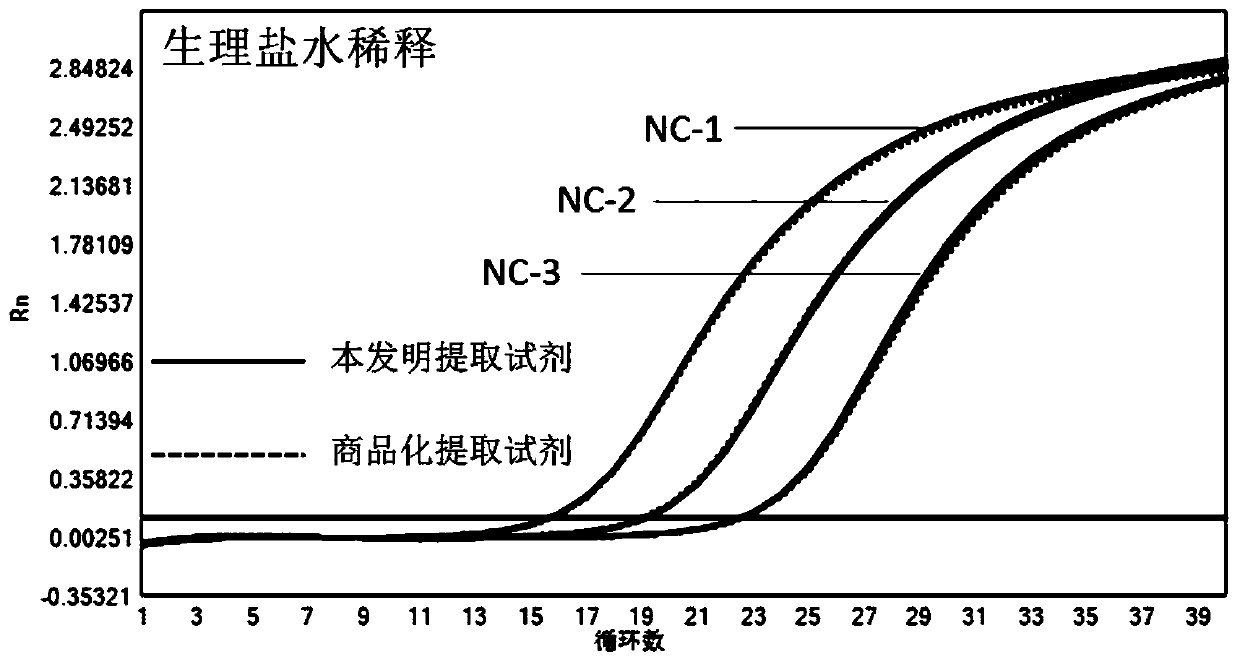
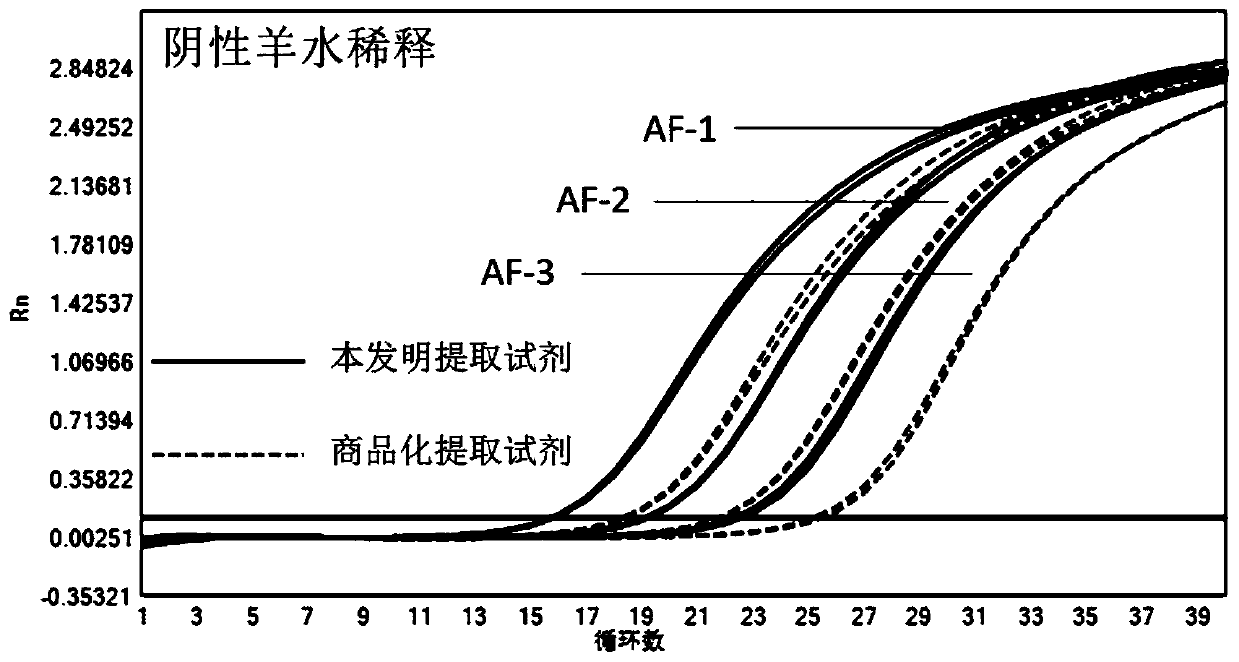
[0092] A high-concentration HCMV-positive amniotic fluid sample was taken, and three gradients were diluted with the HCMV-negative amniotic fluid sample and normal saline respectively. Three concentration samples of HCMV-negative amniotic fluid sample dilution are defined as AF-1, AF-2, AF-3, three concentration samples of physiological saline dilution are respectively defined as NC-1, NC-2, NC-3.
[0093] 1.2 Sample nucleic acid extraction
[0094] Use the magnetic bead method HCMV nucleic acid extraction kit described in Example 1 of the present invention and the comparative extraction reagent (QIAamp circle R DNA mini Kit (QIAGEN company) extraction reagent on the market) to carry out sample extraction, and each extraction is repeated twice. The comparison extraction reagent was operated according to its instructions, and the operation of the kit for extracting HCMV nucleic acid by the magnetic bead method of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap