Method for selectively synthesizing benzyl trifluoromethyl sulfide
A technology of benzyl trifluoromethyl sulfide and selectivity, which is applied in the field of selective synthesis of benzyl trifluoromethyl sulfide, can solve problems such as excess and poor substrate compatibility, and achieves avoiding residues and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
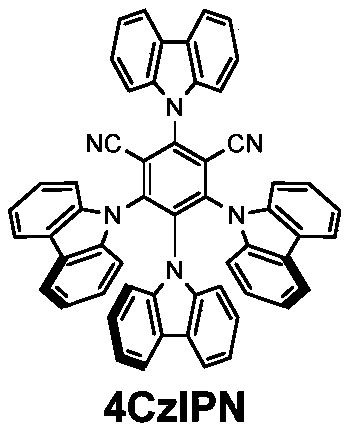
[0052] Weigh in turn (Synthesized according to the above method, the same below) (64.3mg, 0.26mmol), 2-isopentylbenzo[b]thiophene (40.8mg, 0.2mmol), 4CzIPN (provided by Beijing Warwick Chemical Co., Ltd., the same below) (3.2mg, 0.004mmol), K 2 CO 3 (5.52mg, 0.04mmol) was added to the reaction tube, evacuated three times through the vacuum line, and under an argon atmosphere, 4mL of anhydrous acetonitrile was added. Then put it under the irradiation of two 45W blue lights (Kessil, A360NE / WE, the same below), and react at room temperature for 12h. Dry loading, column chromatography (300-400 mesh chromatography silica gel) to obtain 44.4 mg of product, (Eluent: n-hexane), the yield is 73%, 1 H NMR(400MHz, CDCl 3 )δ7.81-7.76(m,1H), 7.70(dd,J=6.9,1.9Hz,1H), 7.32(pd,J=7.1,1.4Hz,2H), 7.22(s,1H), 4.74(dd ,J=9.5,6.5Hz,1H),1.97(ddd,J=14.9,9.4,5.8Hz,1H),1.87(ddd,J=14.1,8.2,6.5Hz,1H),1.68(ddq,J=12.8 ,8.3,6.6Hz,1H),0.94(dd,J=6.6,5.6Hz,6H); 19 F NMR(376MHz, CDCl 3 )δ-39.98; 13 C NMR(101M...
Embodiment 2
[0054] Weigh first (64.3mg, 0.26mmol), 4CzIPN (3.2mg, 0.004mmol), K 2 CO 3 (5.52mg, 0.04mmol), add to the reaction tube, ventilate three times through the vacuum line, under argon atmosphere, add 4mL anhydrous acetonitrile, and then carefully add (37.7mg, 0.2mmol), then placed under the irradiation of two 45W blue light, and react at room temperature for 12h. Dry loading, column chromatography (300-400 mesh chromatography silica gel) to obtain 44.2mg of product, (Eluent: n-hexane), the yield is 76%, 1 H NMR(400MHz, CDCl 3 )δ7.52(dd,J=7.4,1.3Hz,1H),7.47(dd,J=8.2,0.9Hz,1H),7.28(td,J=8.3,7.8,1.5Hz,1H),7.22(td ,J=7.5,1.1Hz,1H),6.63(s,1H),4.54(dd,J=9.3,6.8Hz,1H),2.08(ddd,J=15.0,9.2,6.2Hz,1H),1.85( dt, J = 14.0, 7.4 Hz, 1H), 1.65 (dp, J = 13.2, 6.6 Hz, 1H), 0.95 (dd, J = 6.6, 5.4 Hz, 6H); 19 F NMR(376MHz, CDCl 3 )δ-40.11; 13 C NMR(101MHz, CDCl 3 )δ155.4,154.9,130.4(q,J=307.4Hz),128.0,124.5,123.0,121.0,111.3,104.5,42.4,41.1(d,J=1.9Hz),25.8,22.4,21.7.HRMS(EI)calcd for C 14 H 15 F 3 ...
Embodiment 3
[0056] Weigh first (64.3mg, 0.26mmol), 4CzIPN (3.2mg, 0.004mmol), K 2 CO 3 (5.52mg, 0.04mmol), add to the reaction tube, ventilate three times through the vacuum line, under argon atmosphere, add 4mL anhydrous acetonitrile, and then carefully add (40.3mg, 0.2mmol), then placed under two 45W blue lights, and reacted at room temperature for 12h. Dry loading, column chromatography (300-400 mesh chromatography silica gel) to obtain 34.2mg of product, (Eluent: volume ratio, petroleum ether 60-90: ethyl acetate = 20:1), the yield is 57%, 1 H NMR(400MHz, CDCl 3 )δ8.40(d,J=8.6Hz,1H),7.49(d,J=1.8Hz,1H),7.43(d,J=3.8Hz,1H), 7.29(dd,J=8.6,1.9Hz, 1H), 6.62 (dd, J = 3.8, 0.8 Hz, 1H), 4.31 (dd, J = 9.1, 6.2 Hz, 1H), 2.63 (s, 3H), 2.05 (ddp, J = 30.5, 14.8, 7.9, 7.3Hz, 2H), 0.91 (t, J=7.3Hz, 3H); 19 F NMR(376MHz, CDCl 3 )δ-39.74; 13 CNMR(101MHz, CDCl 3 )δ168.5,135.8,135.0,130.7,130.6(q,J=308.4Hz),125.9,124.6,119.8,116.8,109.1,51.5,30.1,23.9,12.0.HRMS m / z(ESI)calcdfor C 14 H 14 F 3 NNaOS + (M...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap