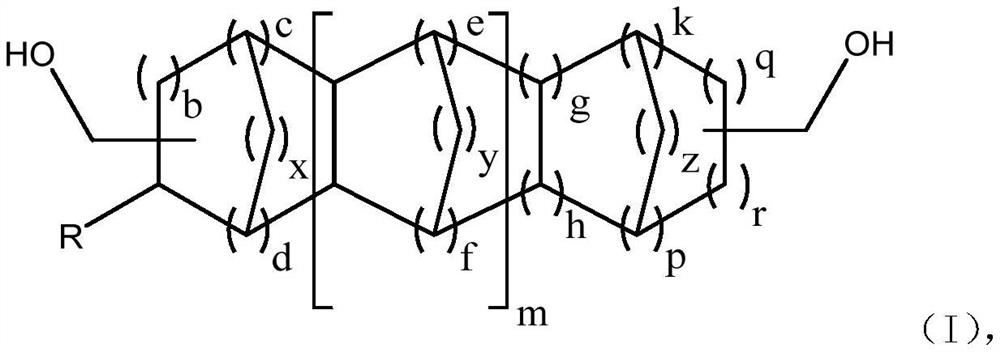
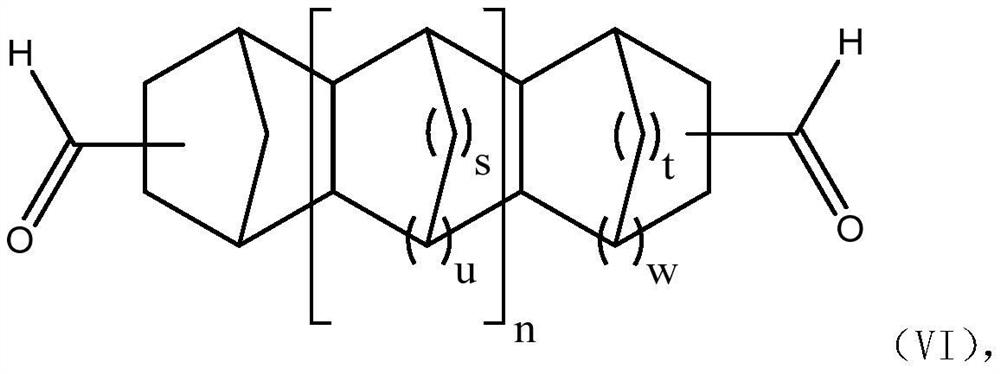
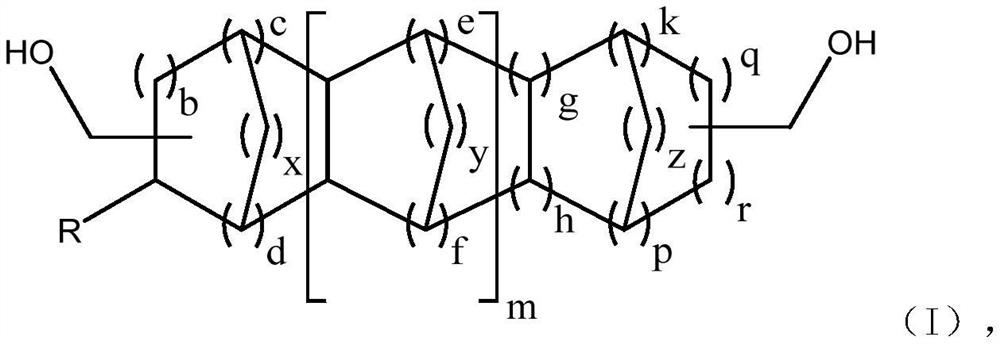
Extraction method for separation of high boiling aldehyde products and recovery of catalyst
一种高沸点、氢甲酰化催化剂的技术,应用在分离方法、物理/化学过程催化剂、溶剂萃取等方向,能够解决生产力不佳、二醛产率降低、缩醛无法通过蒸馏分离等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Example 1: Procedure for the Hydroformylation Process
[0264] The following procedure was performed under an atmosphere with a hydrogen to carbon monoxide ratio of 1:1. 100 grams of methylcyclohexane, 0.015 grams of Rh (acac) (CO) 2 (available from Aldrich) and 4.5 grams of tris(2,4-di-tert-butylphenyl)phosphite (available from BASF) were mixed together to form a hydroformylation mixture. The hydroformylation mixture was heated to 70° C. for 1.5 hours to dissolve uniformly. When the procedure was complete, the hydroformylation mixture was poured into an autoclave at a pressure of 1 kg / cm2 gauge. Thereafter, the temperature and pressure of the autoclave were increased to 80° C. and 50 kg / cm2 gauge, respectively. 50 grams of dicyclopentadiene (available from Zeon) was continuously fed to the autoclave at a rate of 8.3 grams / minute during 30 minutes using a metering pump. The reaction was carried out for 12 hours at a pressure of 50 catties / cm2 gauge. After the react...
Embodiment 2
[0267] Example 2: Procedure of the extraction method
[0268] The non-aqueous hydroformylation product solution (174.5 g) was mixed with an aqueous extraction solvent (MPO: 104.4 g, methanol: 35.0 g, water: 35.1 g) at a mass ratio of 1:1 and the temperature was maintained at 30 ° C. Stir in the vessel. After stirring for 1 hour, the mixture was poured into a glass extractor for 2 hours, and a biphasic mixture (non-aqueous hydroformylation solvent layer (i.e., non-polar phase) and aqueous extraction solvent layer (i.e., polar phase) was obtained. sexuality)). Gas chromatography (Agilent 6980N, column: HP-1 methylsiloxane 30.0 m x 0.32 mm x 1 micron), graphite furnace atomic absorption spectrometer (PerkinElmerAnalyst 600), and inductively coupled plasma atomic emission spectrometer (Agilent) measured the non-polar phase and the polar phase, and the results are shown in Table 2.
[0269] Table 2
[0270]
[0271] a Measured by gas chromatography
[0272] b Measured by ...
Embodiment 3a to 3h
[0293] Examples 3a to 3h: Extraction solvents
[0294] The non-aqueous hydroformylation product solution prepared according to the procedure of Example 1 - Hydroformylation method was extracted using various extraction solvents, as shown in Table 3.
[0295] table 3
[0296]
[0297] In the present invention, partition coefficient Kp=(concentration of high-boiling aldehyde compound in polar phase / concentration of high-boiling aldehyde compound in non-polar phase). For example, Kp=(Concentration of TCDDA in polar phase / Concentration of TCDDA in non-polar phase).
[0298] For example, TCDDA is the high boiling aldehyde compound; the polar phase is the aqueous extraction solvent layer; and the non-polar phase is the non-aqueous hydroformylation solvent layer.
[0299] The extraction solvents of Table 3 (Examples 3a to 3d) are each embodiments of the extraction solvents (ie, aqueous extraction solvents) of the present invention. None of the extraction solvents of Table 3 (Ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com