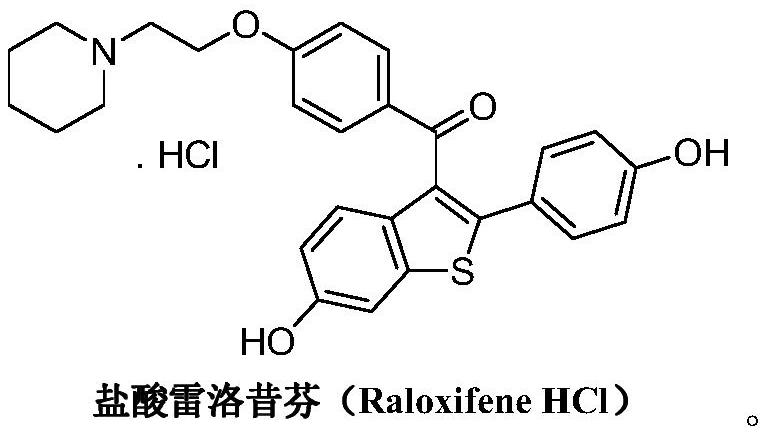
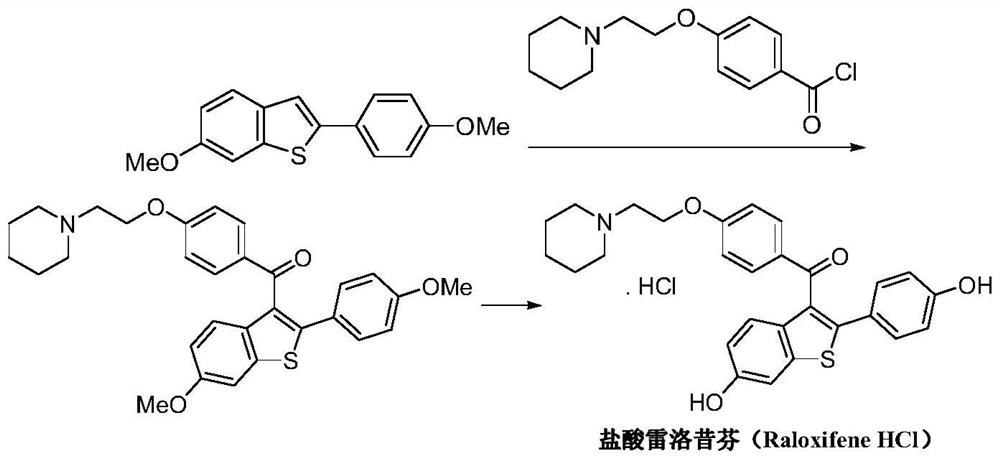
A kind of preparation method of raloxifene hydrochloride and its intermediate
A technology for raloxifene hydrochloride and an intermediate, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of difficult preparation of starting materials, unfavorable industrial production, and large environmental impact, and achieves the advantages of controlling impurities, improving atom utilization, Less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A) Preparation of 1-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-2-(2-benzylthio-4-methoxyphenyl)ethanone (formula 3) :
[0065] (3-Methoxyphenyl) benzyl sulfide (50.0g, Formula 5) was dissolved in acetonitrile (700mL), cooled in an ice bath, and periodic acid (75.0g) and ferric chloride (1.1g, 6.8 mmol), reacted at 25°C for 6h, evaporated to dryness under reduced pressure, extracted with dichloromethane, washed with saline and 5% sodium thiosulfate solution successively, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure, the obtained sulfide The crude sulfone compound (Formula 4) was mixed with 1-[2-(4-ethynylphenoxy)ethyl]piperidine (50.0g) and trifluoromethanesulfonic acid (163g, 1.09mol), and reacted at 70°C for 6h. After the reaction is completed, the reaction mixture is post-treated, and recrystallized from a mixed solvent of ethyl acetate-petroleum ether to obtain 1-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-2-(2- Benzylthio-4-methoxypheny...
Embodiment 2
[0073] A) Preparation of 1-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-2-(2-benzylthio-4-methoxyphenyl)ethanone (formula 3) :
[0074] (3-Methoxyphenyl) benzyl sulfide (65.0g, Formula 5) was dissolved in toluene (850mL), cooled in an ice bath, and periodic acid (170.0g) and ferric chloride (0.9g, 5.5 mmol), reacted at 27°C for 4h, evaporated to dryness under reduced pressure, extracted with dichloromethane, washed with saline and 5% sodium thiosulfate solution successively, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure, the obtained sulfide The crude sulfone compound (Formula 4) was mixed with 1-[2-(4-ethynylphenoxy)ethyl]piperidine (43.0g) and trifluoromethanesulfonic acid (422g, 2.8mol), and reacted at 80°C for 4h. After the reaction is completed, the reaction mixture is post-treated, and recrystallized from a mixed solvent of ethyl acetate-petroleum ether to obtain 1-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-2-(2- Benzylthio-4-methoxyphenyl)eth...
Embodiment 3
[0082] A) Preparation of 1-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-2-(2-benzylthio-4-methoxyphenyl)ethanone (formula 3) :
[0083] (3-Methoxyphenyl) benzyl sulfide (300.0g, formula 5) was dissolved in methyl tert-butyl ether (4000mL), cooled in an ice bath, and periodic acid (700.0g, 3.07mol) and three Ferric chloride (5.0g, 0.03mol), reacted at 30°C for 2h, rotary evaporated to dryness under reduced pressure, extracted with dichloromethane, washed with saline and 5% sodium thiosulfate solution successively, dried over anhydrous sodium sulfate, and dried under reduced pressure Rotary evaporation to dryness, the obtained sulfoxide compound (formula 4) crude product was mixed with 1-[2-(4-ethynylphenoxy)ethyl]piperidine (230.0g), trifluoromethanesulfonic acid (1500.0g, 10mol ) mixed, reacted at 100°C for 2h, after the reaction was completed, the reaction mixture was post-treated, and recrystallized from a mixed solvent of ethyl acetate-petroleum ether to obtain 1-{4-[2-(piperidin-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com