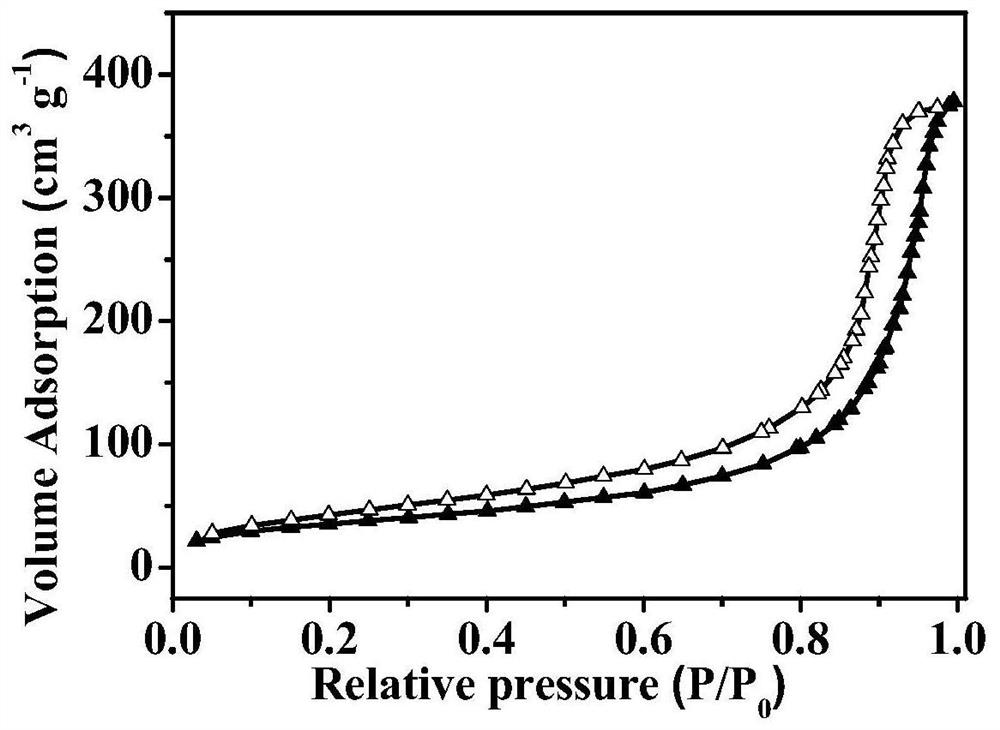
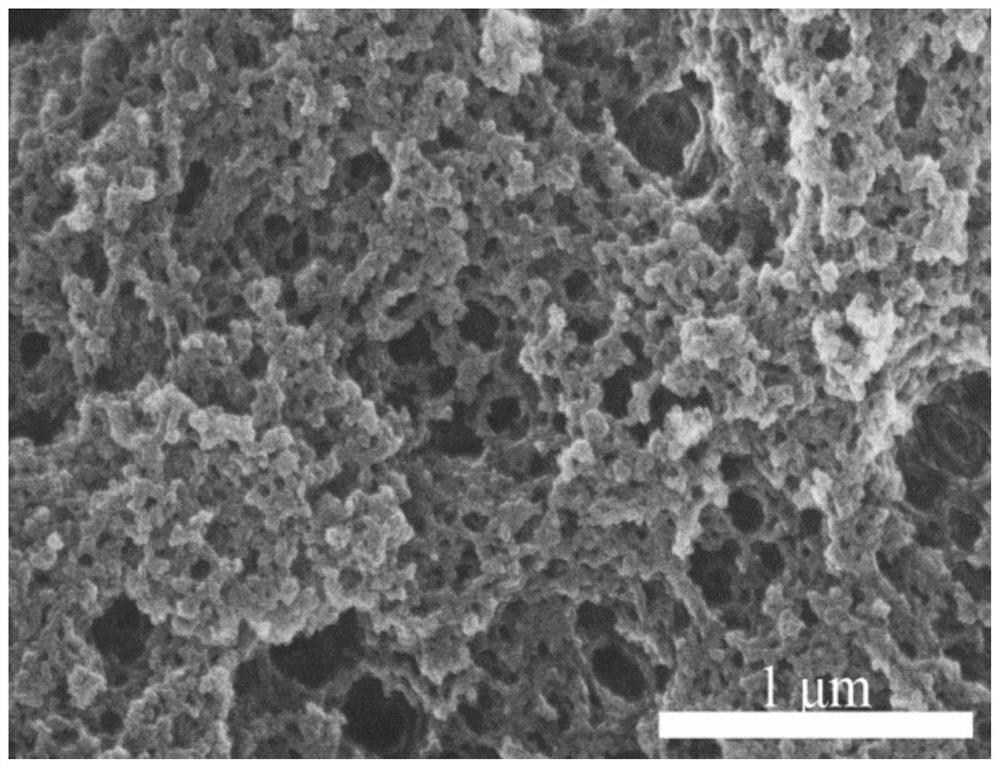
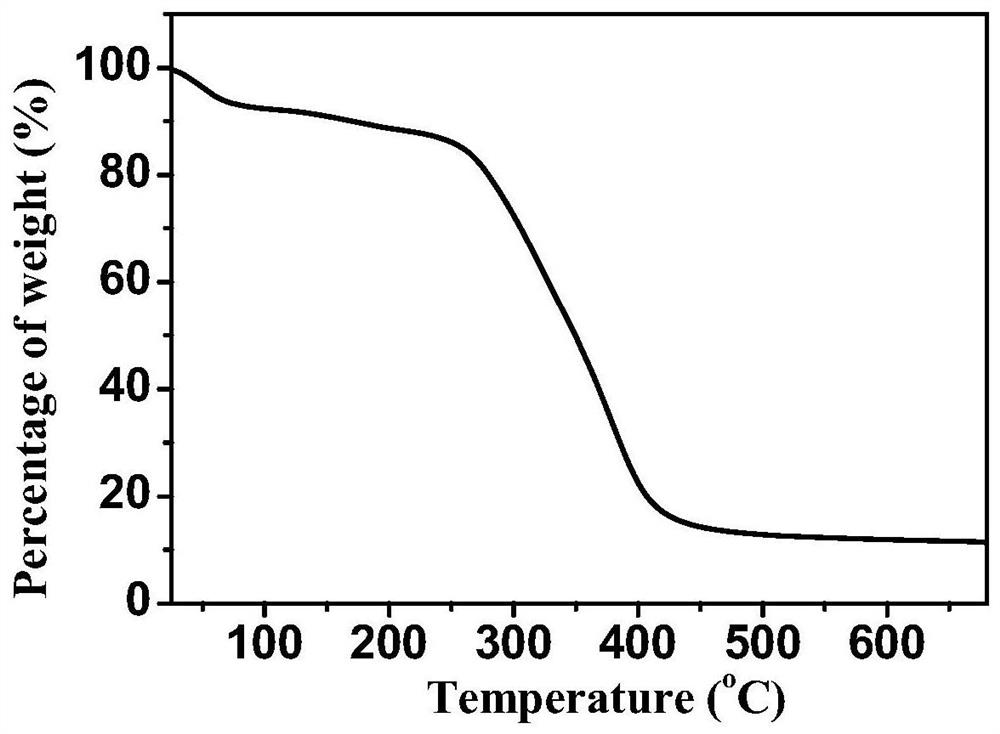
A nitrogen heterocyclic carbene/co 2 Adduct functional organic porous polymer, preparation method and application
A porous polymer, nitrogen heterocyclic carbene technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problem of easy deactivation, unstable solid base, The problem of unsatisfactory catalyst recovery and reuse effect, to achieve the effect of simple operation, rich adduct active sites, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]1), preparation of organic porous polymer PEM1 containing imidazole functional group
[0035] The preparation route is:
[0036]
[0037] Dissolve N-vinylimidazole (0.94g, 10mmol), ethylene glycol dimethacrylate (2.0g, 10mmol) and azobisisobutyronitrile (AIBN, 0.08g) in 5mL toluene, and stir at room temperature for 2h Transfer to a 25mL polytetrafluoroethylene-lined stainless steel autoclave, put it in an oven at 90°C for solvothermal polymerization for 24 hours, after cooling to room temperature, evaporate the solvent at room temperature, and use ethanol and deionized Washed twice with water and dried in an oven at 100°C for 24 hours to obtain the organic porous polymer PEM1 containing imidazole functional groups.
[0038] 2), azacyclic carbene / CO 2 Preparation of Adduct Functionalized Organic Porous Polymer PEMC1
[0039] The preparation route is:
[0040]
[0041] Take 1g of the above-mentioned polymer PEM1 and disperse it in 5g of dimethyl carbonate, stir a...
Embodiment 2
[0047] 1), preparation of organic porous polymer PEM2 containing imidazole functional group
[0048] N-vinylimidazole (0.94g, 10mmol), ethylene glycol dimethacrylate (4.0g, 20mmol) and 2,2'-azobis(2-methylpropylamidine) dihydrochloride ( 0.4g) was dissolved in 10mL tetrahydrofuran, stirred at room temperature for 3h, then transferred to a 25mL polytetrafluoroethylene-lined stainless steel autoclave, and placed in a 100°C oven for solvothermal polymerization for 36h. After cooling to room temperature, the solvent was evaporated at room temperature. The obtained white monolith was mechanically crushed, washed three times with ethanol and deionized water, and dried in an oven at 80° C. for 30 h to obtain an organic porous polymer PEM2 containing imidazole functional groups.
[0049] 2), azacyclic carbene / CO 2 Preparation of Adduct Functionalized Organic Porous Polymer PEMC2
[0050] Take 1g of the above-mentioned polymer PEM2 and disperse it in 10g of dimethyl carbonate, stir a...
Embodiment 3
[0052] 1), preparation of organic porous polymer PEM3 containing imidazole functional group
[0053] Dissolve N-vinylimidazole (0.94g, 10mmol), ethylene glycol dimethacrylate (6.0g, 30mmol) and benzoyl peroxide (0.5g) in 10mL of benzene, stir at room temperature for 3h and transfer to 25mL In a stainless steel autoclave lined with polytetrafluoroethylene, put it in an oven at 110°C for solvothermal polymerization for 12 hours. After cooling to room temperature, evaporate the solvent at room temperature. The obtained white monolith is mechanically broken and washed with ethanol and deionized water respectively. Twice, drying in an oven at 120° C. for 12 hours to obtain an organic porous polymer PEM3 containing imidazole functional groups.
[0054] 2), azacyclic carbene / CO 2 Preparation of Adduct Functionalized Organic Porous Polymer PEMC3
[0055] Take 1g of the above-mentioned polymer PEM3 and disperse it in 15g of dimethyl carbonate, stir at room temperature for 2 hours, tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com