A kind of preparation method of β-trifluoromethyl alcohol catalyzed by visible light
A technology of trifluoromethyl alcohol and visible light, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylate esters, etc., can solve the problems that do not conform to the development trend of organic chemistry, do not conform to green chemistry, and accelerate energy consumption. , to achieve the effect of wide substrate application, low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Add 1a (0.2mmol, 46.5mg), 2a (0.8mmol, 131.5mg), Mn(acac) once in the test tube 3 (0.02mmol, 7.3mg), 1,4-dioxane (3.0mL) and acetone (1.0mL). Then the system was stirred for 24 hours under 40W blue light LED irradiation and room temperature in the air, then directly added 2.0mL ethyl acetate to dilute, removed the solvent with a rotary evaporator, adsorbed on silica gel, and obtained the product 3a through simple column chromatography. The yield is 80%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0042] 1 H NMR (400MHz, CDCl 3 )δ7.97–7.94(m,2H),7.45–7.47(m,2H),4.65–4.59(m,1H),4.42–4.37(m,1H),4.20–4.14(m,1H),2.99( s,1H),2.52–2.19(m,2H),2.08-1.97(m,1H),1.93–1.85(m,1H). 13 C NMR (100MHz, CDCl 3)δ167.06, 156.96, 129.47, 126.93, 126.14, (q, J=276Hz), 124.76, 62.96 (q, J=3Hz), 61.01, 41.11, (q, J=27Hz), 36.31, 35.05, 31.02. 19 F...
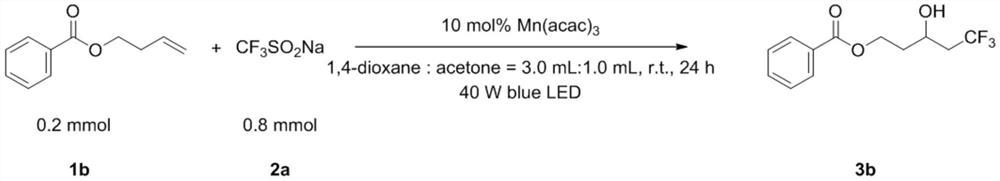
Embodiment 2
[0044]
[0045] Add 1b (0.2mmol, 35.3mg), 2a (0.8mmol, 131.5mg), Mn(acac) once in the test tube 3 (0.02mmol, 7.3mg), 1,4-dioxane (3.0mL) and acetone (1.0mL). Then the system was stirred for 24 hours under 40W blue light LED irradiation and room temperature in the air, then directly added 2.0mL ethyl acetate to dilute, removed the solvent with a rotary evaporator, adsorbed on silica gel, and obtained the product 3b through simple column chromatography. The yield was 75%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0046] 1 H NMR (400MHz, CDCl 3 )δ8.03(d, J=8.0Hz, 2H), 7.58(t, J=16.0Hz, 1H), 7.45(t, J=16.0Hz, 2H), 4.68–4.62(m, 1H), 4.44– 4.39(m,1H),4.21–4.16(m,1H),2.70(s,1H),2.47–2.23(m,2H),2.07-1.99(m,1H),1.95–1.87(m,1H). 13 C NMR (100MHz, CDCl 3 )δ167.00, 133.23, 129.76, 129.60, 128.46, 126.14, (q, J = 276Hz), 63.04 (q, J = 3Hz), 61.22, 41...
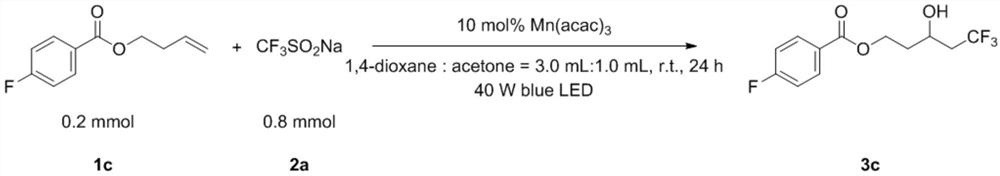
Embodiment 3
[0048]
[0049] Add 1c (0.2mmol, 38.9mg), 2a (0.8mmol, 131.5mg), Mn(acac) once in the test tube 3 (0.02mmol, 7.3mg), 1,4-dioxane (3.0mL) and acetone (1.0mL). Then the system was stirred for 24 hours under 40W blue light LED irradiation and room temperature in the air, then directly added 2.0mL ethyl acetate to dilute, removed the solvent with a rotary evaporator, adsorbed on silica gel, and obtained the product 3c through simple column chromatography. The yield was 76%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0050] 1 H NMR (400MHz, CDCl 3 )δ8.06–8.03(m,2H),7.12(t,J=16.0Hz,2H),4.66–4.60(m,1H),4.44–4.38(m,1H),4.20–4.15(m,1H) ,2.65(s,1H),2.49–2.23(m,2H),2.06-1.98(m,1H),1.95–1.86(m,1H). 13 C NMR (100MHz, CDCl 3 ( q, J=3Hz), 61.34, 41.19 (q, J=26Hz), 36.24. 19 F NMR (376MHz, CDCl 3 )δ=-63.47(s,3F),-105.10(s,1F).HRMS(ESI-TOF):Anal.Calcd....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com