A fully human monoclonal antibody against complement c5 molecule and its application
A monoclonal antibody and anti-complement technology, applied in the field of peptides, can solve problems such as the controversy over the efficacy of eculizumab, and achieve the improvement of symptoms such as vasculitis and crescent/necrosis, improvement of survival rate, and excellent antigen-binding activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
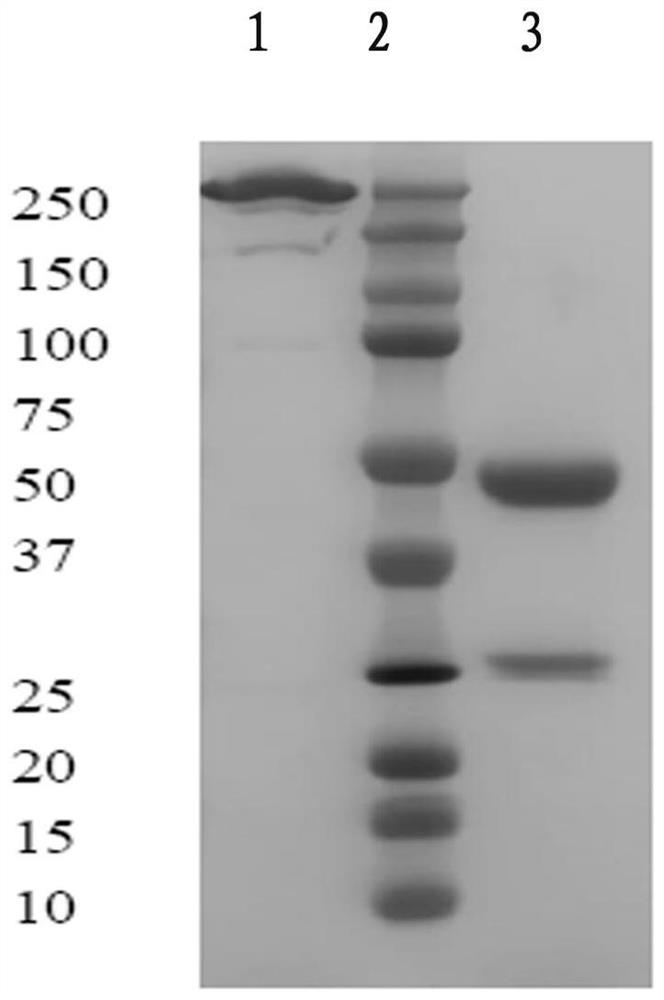
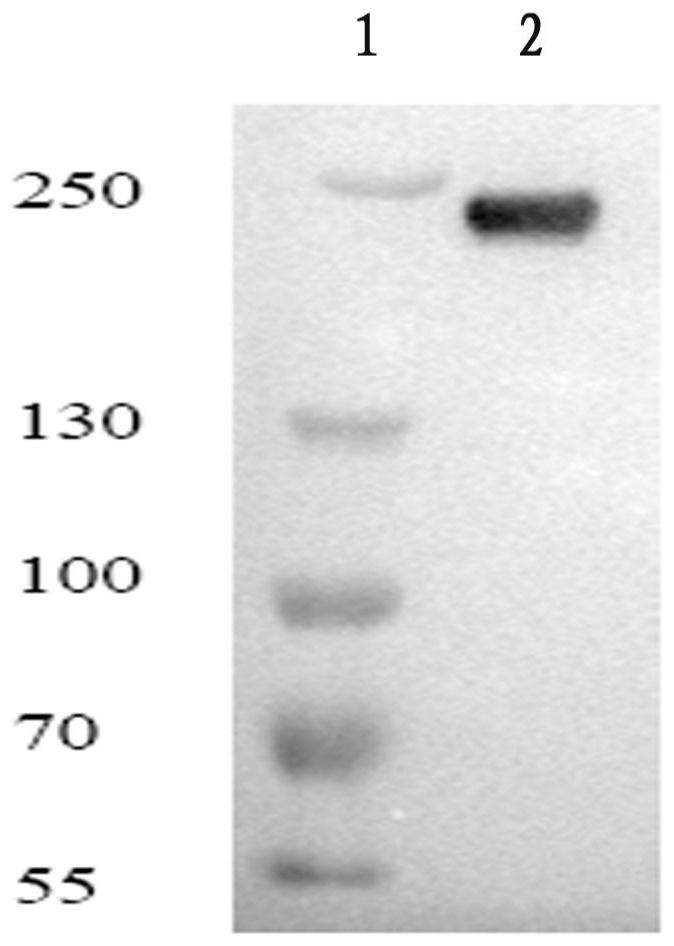
[0026] Example 1. Preparation of fully human monoclonal antibody against complement C5 molecule
[0027] 1.1 Construction and expression of phage antibody expression library Refer to Example 1 of Chinese invention patent application CN109575132A. This application is incorporated by reference, and the disclosure content of CN109575132A is incorporated into the specification of this application as a part of this application.
[0028] 1.2 Screening of recombinant phage antibody: Coat polyethylene culture dish with C5 antigen (Complement Technology, I product number: A120Lot#, Accession#P06684), and incubate the supernatant containing recombinant phage with the culture dish at 37°C for 2 hours. Wash the plate 20 times with PBS, then wash the plate 20 times with PBST (PBS containing 0.05% Tween 20), discard the PBST. Add 10 mL of TG1 cells in logarithmic growth phase and incubate at 37°C for 1 hour. After centrifugation, the supernatant was collected for the next round of screenin...
Embodiment 2
[0059] Example 2. Kinetic analysis of the interaction between anti-complement C5 monoclonal antibody and C5 ligand
[0060] The kinetic analysis of the interaction between anti-complement C5 monoclonal antibody and C5 ligand was detected by surface plasmon resonance (SPR) detection system.
[0061] 2.1 Experimental instruments and reagents
[0062] Instrument: Reichert2SPR (Reichert Company), chip: SAM chip (macromolecular detection), (ReichertInc Company, PART NO: 13206061).
[0063] Reagents: 1xPBST 500ml (filtered, 0.22uM membrane filter), EDC (preparation for current use), NHS (preparation for current use), 1MpH8.5 ethanolamine (5-10ml), 10mM pH2.0 HCl (5-10ml) , 10 mM pH 2.0 Glycine (5-10 ml).
[0064] 2.2. Experimental steps
[0065] 2.2.1 Pre-enrichment
[0066] 2.2.1.1 Dilute the protein with different pH sodium acetate to 10 μg / mL, 200 μL.
[0067] Table 1. Sodium acetate pH selection table
[0068] protein name Sodium acetate pH fixed channel ...
Embodiment 3
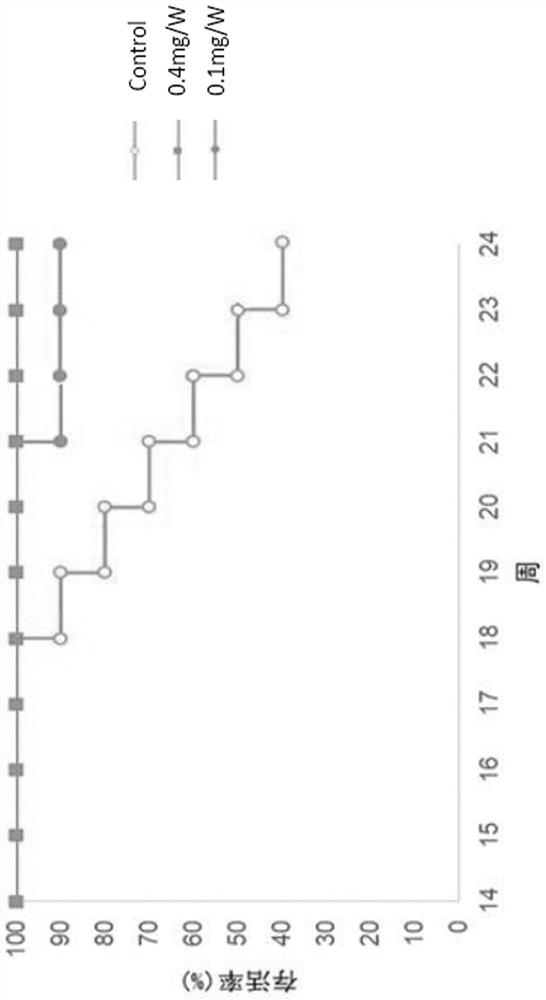
[0085] Example 3. In vitro experiment of anti-C5 whole antibody inhibiting complement activation
[0086] To measure the inhibitory activity on complement, 60%-80% confluent CHO cells were separated with ethylenediaminetetraacetic acid, washed twice with DMEM, and then resuspended in DMEM to make the final concentration of 10 6 cells / mL. Add 100mL / L rabbit anti-CHO cell membrane antiserum to the cell suspension and react at 4°C for 30min to sensitize the cells. Then the antiserum was discarded, and the cells were resuspended in NHS diluted with DMEM to a final volume of 50 μL or 100 μL. After 60 minutes at 37°C, the cell viability was measured by placenta blue staining and exclusion method (both live and dead cells were counted). Monoclonal antibody diluted with DEME was first added to NHS, and then added to CHO cell suspension. The final concentration was based on the control CHO cells lysed by 100 g / L NHS which can cause about 90% antibody sensitization. Complement-media...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com