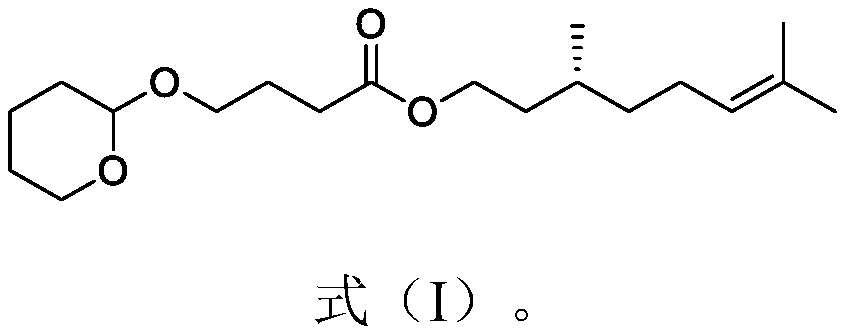
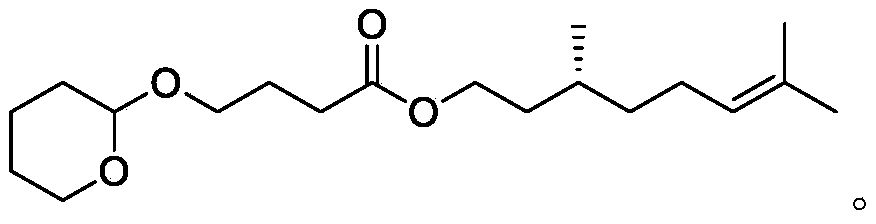
Citronellyl ester compound, preparation method thereof and spice
A compound, a technology of citronellyl ester, applied in the field of spices, citronellyl ester compounds and preparation methods thereof, can solve the problems of singleness and can not meet the needs of consumers and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
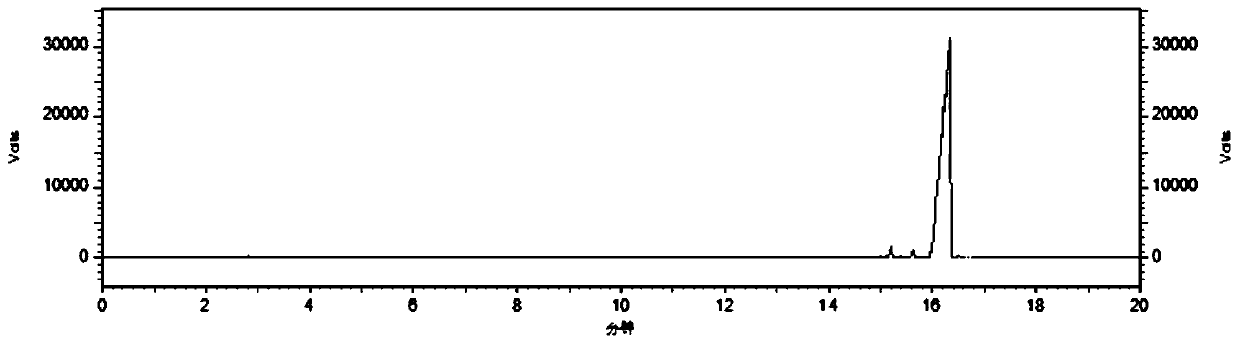
Image
Examples
preparation example Construction
[0028] The preparation method of the citronellyl ester compound of above-mentioned embodiment, comprises the following steps:
[0029] S110: Under the condition of continuously feeding anhydrous hydrogen chloride gas, mixing 5-hydroxyvaleraldehyde and chloroform to perform a hemiacetal reaction to obtain 2-hydroxytetrahydropyran;
[0030] Wherein the structural formula of 5-hydroxypentanal is The structural formula of 2-hydroxytetrahydropyran is
[0031] The chemical reaction equation of step S110 is as follows:
[0032]
[0033] Wherein, chloroform is used as a solvent to dissolve 5-hydroxypentanal, and anhydrous hydrogen chloride is used as a catalyst.
[0034] Specifically, the above hemiacetal reaction is carried out at a reaction temperature of 50° C. to 70° C. for 2 hours to 4 hours. In a specific example, the reaction temperature may be 50°C, 55°C, 60°C or 70°C, and the reaction time may be 2h, 3h or 4h. Appropriate reaction temperature and time can make the r...
Embodiment 1
[0080] The preparation method of the citronellyl compound of the present embodiment may further comprise the steps:
[0081] 1) Preparation of 2-hydroxytetrahydropyran
[0082] Add 51g of 5-hydroxyvaleraldehyde and 100mL of chloroform to the three-necked flask, while continuously feeding anhydrous hydrogen chloride gas, heating to 60°C, and reacting for 3h; the reacted product is distilled under reduced pressure and collected under a pressure of 300Pa The mother liquor temperature is 50 ℃~70 ℃ distillate, obtains 46g colorless transparent viscous oil product 2-hydroxytetrahydropyran;
[0083] 2) Preparation of 4-(2-oxyl-tetrahydropyran)butanol
[0084] 46g 2-hydroxytetrahydropyran, 41g 1,4-butanediol, 125mL tetrahydrofuran (THF), 153g triphenylphosphine (PPh 3 ), and cooled to 0°C, slowly add 136g of diisopropyl azodicarboxylate dropwise, and stir the reaction at room temperature for 12h after the dropwise addition; the product after the reaction is subjected to vacuum disti...
Embodiment 2
[0097] The preparation method of the citronellyl compound of the present embodiment may further comprise the steps:
[0098] 1) Preparation of 2-hydroxytetrahydropyran
[0099] Add 45g of 5-hydroxyvaleraldehyde and 900mL of chloroform to the three-necked flask, while continuously feeding anhydrous hydrogen chloride gas, heating to 70°C, and reacting for 2h; the reacted product is distilled under reduced pressure and collected under a pressure of 300Pa The mother liquor temperature is 50 ℃~70 ℃ distillate, obtains 37g colorless transparent viscous oil product 2-hydroxytetrahydropyran;
[0100] 2) Preparation of 4-(2-oxyl-tetrahydropyran)butanol
[0101] Add 37g 2-hydroxytetrahydropyran, 32g 1,4-butanediol, 100mL tetrahydrofuran THF, 148g triphenylphosphine (PPh 3 ), and cooled to 2°C, slowly added dropwise 144g of diisopropyl azodicarboxylate, stirred and reacted at room temperature for 10h after the dropwise addition; the product after the reaction was distilled under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com