A recombinant expression vector expressing ll-37 polypeptide, recombinant Lactococcus lactis, antiviral drug, construction method and application
A technology of Lactococcus lactis and LL-37, which is applied in the field of genetic engineering, can solve the problems of low expression efficiency of LL-37, limit the application of LL-37, and high production cost, so as to avoid low expression efficiency and solve the uncertainty of metabolism in vivo sex, the effect of solving cumbersomeness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Construction of PNZ8149-7×LL-37 expression strain
[0041] 1. The complete sequence of SphI-SPusp45-LEISS-DDDDK-7×LL-37-XbaI was chemically synthesized to obtain the pUC57-7×LL-37 vector;
[0042] 2. Construction of PNZ8149-7×LL-37 vector
[0043] 1) Use the Tiangen plasmid mini-extraction kit to extract the pNZ8149, pUC57-7×LL-37 plasmids;
[0044] 2) Enzyme digestion: PNZ8149, pUC57-7×LL-37 enzyme digestion system is shown in Table 1, the total system is 50 μl, 37 ° C enzyme digestion for 3 hours;
[0045] Table 1 PNZ8149, pUC57-7×LL-37 enzyme digestion system
[0046]
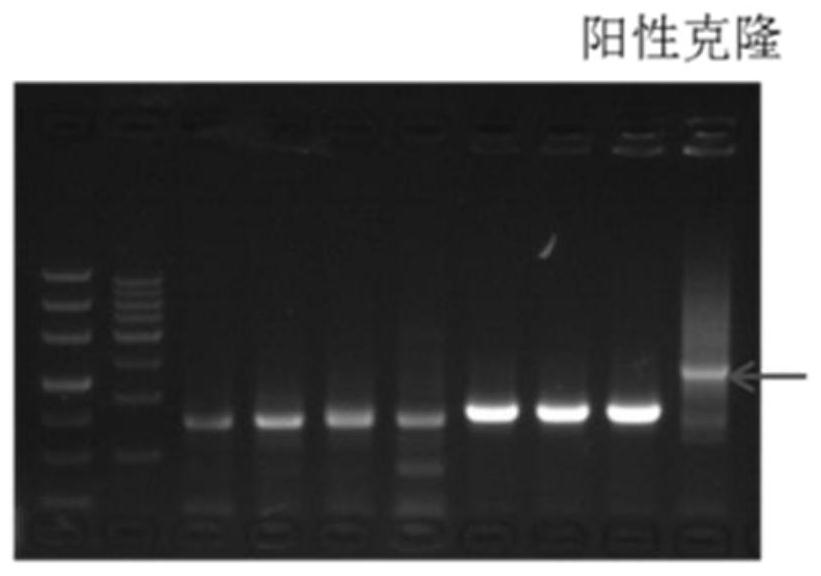
[0047] 3) Preparation of 1% agarose gel electrophoresis, electrophoresis at 120V for 30 minutes, and recovery by cutting the gel;
[0048] 4) Enzyme linkage: the enzyme linkage system is shown in Table 2, the total system is 20 μl, insert fragment:vector = 7:1, overnight at 16°C;
[0049] Table 2 enzyme-linked system
[0050]
[0051] 5) Purification and recovery of the ligated pr...
Embodiment 2
[0052] Example 2 PNZ8149-7×LL-37 Electroporation of Lactococcus lactis NZ3900
[0053] 1. Competent preparation of Lactococcus lactis NZ3900
[0054] 1) Inoculate Lactococcus lactis MG1363 frozen at -80°C into 5ml of M17 liquid medium containing 5% glucose, and culture overnight at 30°C;
[0055] 2) Inoculate the obtained bacterial liquid at 1% in M17 liquid medium containing 2.5% Gly and 5% glucose, culture it statically at 30°C until the OD600 value of the bacterial cell is 0.3-0.4, and collect it for later use;
[0056] 3) Put the above-collected bacterial culture in an ice bath for 10 minutes, centrifuge at 5000 rpm at 4°C for 5 minutes; collect the bacterial cells;
[0057] 4) The precipitate was washed twice with 1 / 10 volume of ice-cold 10% sucrose and 10% glycerol mixed solution, centrifuged at 8000 rpm at 4°C for 5 min, and the precipitate was collected;
[0058] 5) Finally, the precipitate was resuspended in a mixed solution of 1 / 100 volume 10% sucrose and 10% glyce...
Embodiment 3
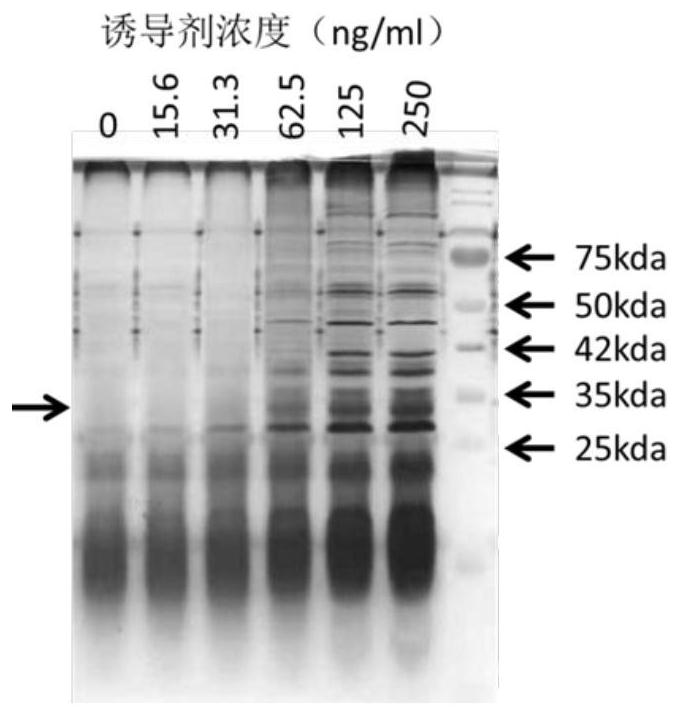
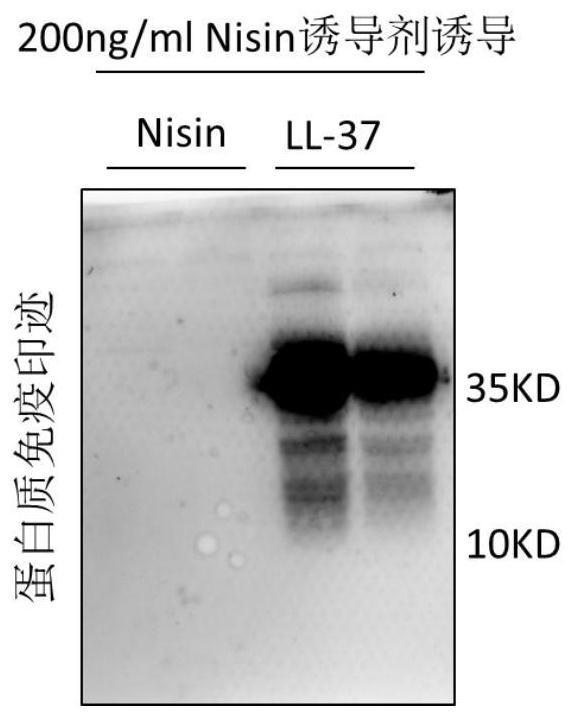
[0068] Example 3 Silver staining and Western detection of the protein expression ability of the PNZ8149-7×LL-37 expression strain
[0069] 1. Induced secretory expression of PNZ8149-7×LL-37 in Lactococcus lactis
[0070] 1) Introduce the strain into M17 liquid medium, culture it overnight at 30°C, transfer it to a new M17 broth medium the next day, and add the inducer nisin when the OD600 value reaches 0.4-0.6 Nisin continued to culture for 5h;
[0071] 2) Centrifuge at 10,000 rpm for 20 minutes after the culture, take the supernatant, filter the supernatant with a 0.22 μm filter membrane, add N-lauroyl sarcosinate to a final concentration of 0.1%, and keep at room temperature for 15 minutes;
[0072] 3) Add trichloroacetic acid to a final concentration of 7.5%, mix well, and place on ice for 2 hours;
[0073] 4) Centrifuge at 10000rpm for 10min, discard the supernatant, add 2ml tetrahydrofuran, and centrifuge at 10000rpm for 10min;
[0074] 5) Centrifuge at 10000rpm for 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com