Method for detecting cefixime polymer impurities
A technology of cefixime dimer and cefixime, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of poor peak shape of cefixime polymers, and achieves wide application range and good detection effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 stock solution
[0064] Preparation of diluent solution: 0.075 mol / L anhydrous disodium hydrogen phosphate: 0.075 mol / L anhydrous sodium dihydrogen phosphate=61:39.
[0065] Sensitivity solution preparation: Accurately measure an appropriate amount of the control solution, dilute it with a diluent to make a solution containing about 0.5 mg of cefixime per 1 ml, and use it as a sensitivity solution.
[0066] Preparation of test solution: get cefixime granules (G1802001 batch), add appropriate amount of diluent, dissolve by ultrasonic, dilute with diluent to make a solution containing cefixime 1mg per 1ml, filter, discard 2.0ml First filtrate, get the continued filtrate as need testing solution;
[0067] Preparation of control solution: Accurately measure an appropriate amount of the test solution, and dilute it with a diluent to prepare a solution containing about 1 μg of cefixime per 1 ml, as a control solution.
[0068] Preparation of ...
Embodiment 2
[0069] Embodiment 2 system suitability test
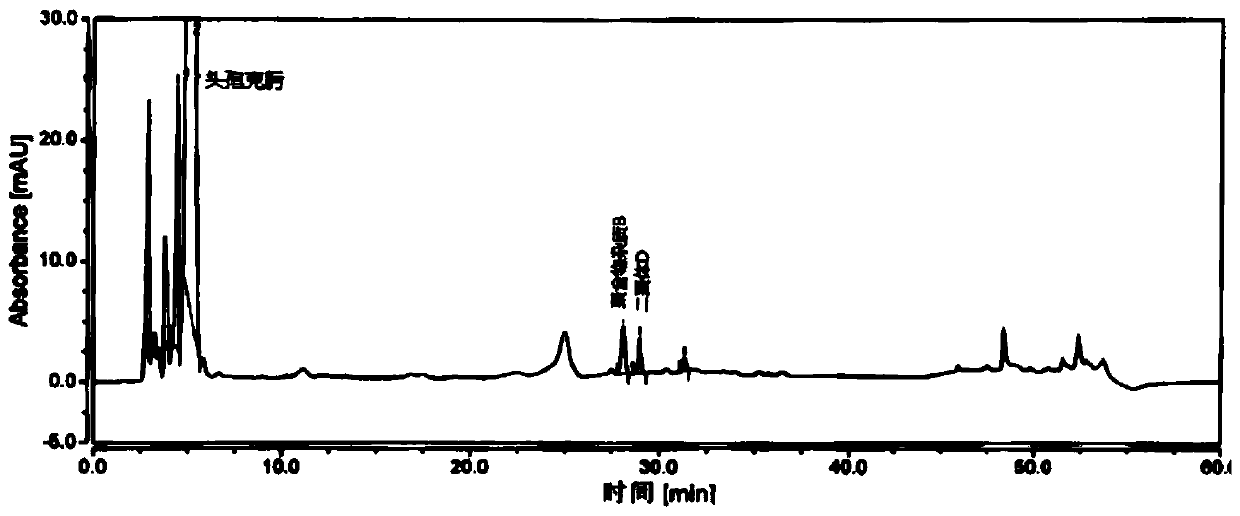
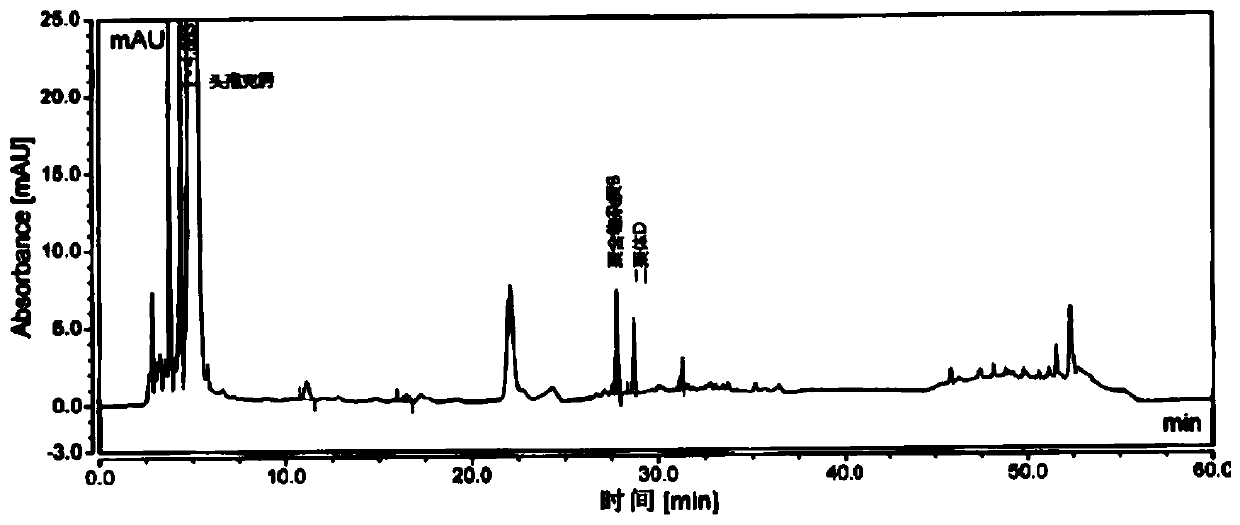
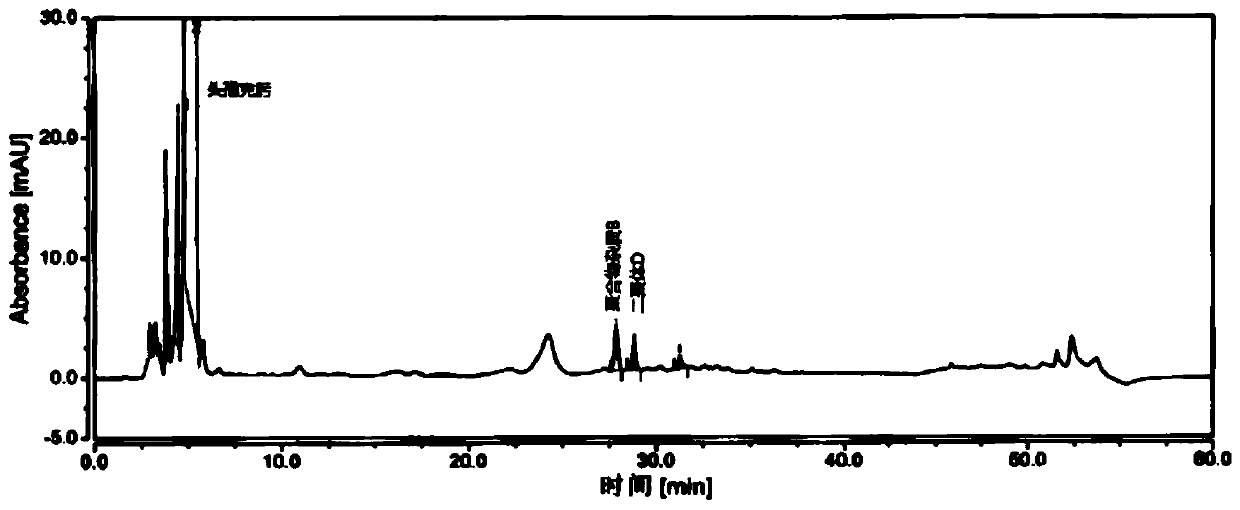
[0070] Take an appropriate amount of cefixime polymer system suitability reference substance, add phosphate buffer (0.075mol / L anhydrous disodium hydrogen phosphate: 0.075mol / L anhydrous sodium dihydrogen phosphate=61:39) to dissolve and dilute to prepare Each 1ml contains about 1mg of cefixime, 2mg of polymer impurity B and 2mg of dimer D, take 20ml and inject it into the liquid chromatograph, record the chromatogram, the separation degree of polymer impurity B and dimer D should meet Require.
Embodiment 3
[0071] The detection of polymer impurity in the cefixime granule of embodiment 3
[0072] Chromatographic column: Hypersil Gold C18, 4.6mm×250mm, 3μm;
[0073] Mobile phase: mobile phase A is 0.1% formic acid solution, and mobile phase B is acetonitrile.
[0074] Flow rate: 1.0ml / min;
[0075] Detection wavelength: 254nm;
[0076] Column temperature: 35°C;
[0077] Injection volume: 20 μL.
[0078] Gradient elution program: as shown in Table 3.
[0079] Table 3 Gradient elution program
[0080]
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com