HUMAN IgG Fc DOMAIN VARIANTS WITH IMPROVED EFFECTOR FUNCTION
A technology of variants and effective doses, applied in the field of human IgGFc domain variants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] This example describes the materials and methods used in Examples 2 to 3 below
[0146] Materials and Methods
[0147] mouse strain
[0148] All mouse in vivo experiments were performed in accordance with federal law and institutional guidelines and were approved by the Institutional Animal Care and Use Committee of Rockefeller University. Mice were bred and maintained at the Comparative Bioscience Center, Rockefeller University. The following strains were used for experiments: (i) FcγR-deficient mice (FcγR 空 ), previously developed and characterized in Smith, P. et al., Pro Natl Acad Sci US A 109, 6181-6186 (2012); (ii) FcγR humanized mouse (mFcγRα 空 , Fcgr1 - / - , hFCGR1A + , hFCGR2A + , hFCGR2B + , hFCGR3A + , hFCGR3B + ), generated and extensively characterized in Smith, P. et al., Pro Natl Acad Sci US A 109, 6181-6186 (2012); (iii) FcγR / FcRn humanized mouse (m FcγRα 空 , Fcgr1 - / - , Fcgrt - / - , hFCGR1A + , hFCGR2A + , hFCGR2B + , hFCGR3A + , hFCGR3B ...
Embodiment 2
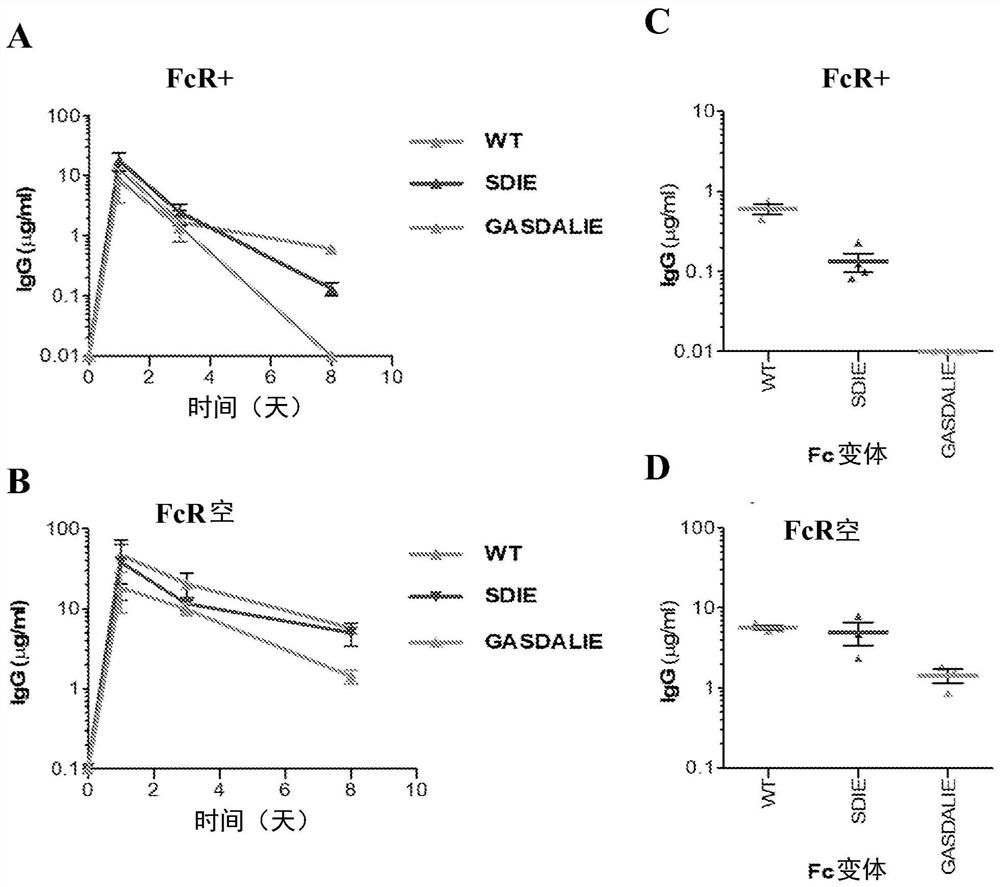
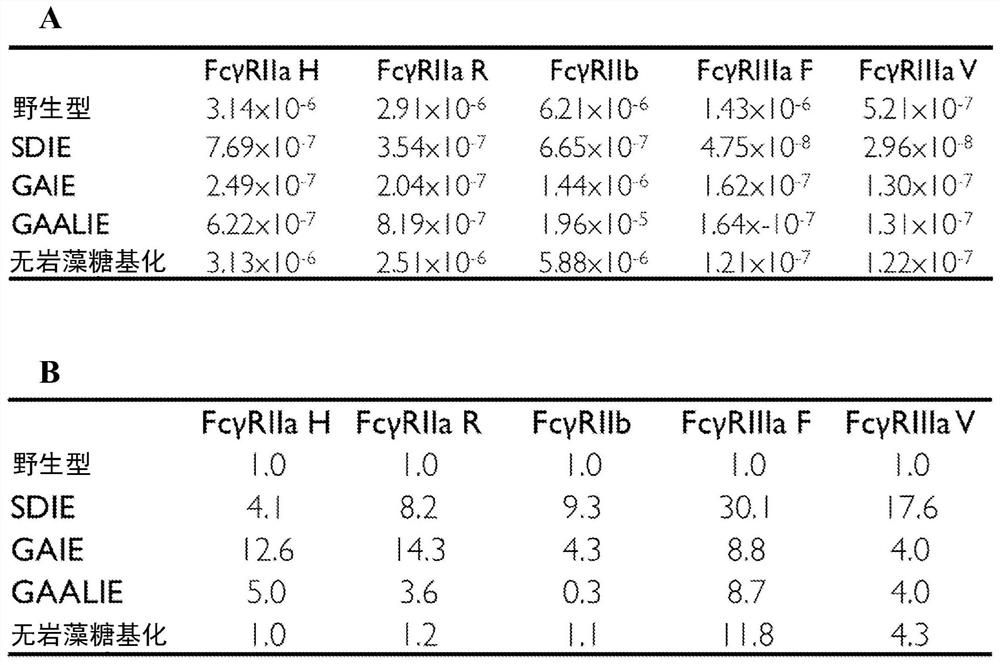
[0158] An Fc domain variant (termed GASDALIE) containing specific mutations (G236A / S239D / A330L / I332E) on the amino acid backbone of human IgGl was developed. It exhibits selectively enhanced binding to human activating FcγRs, FcγRIIa and FcγRIIIa (Smith, P., DiLillo, D.J., Bournazos, S., Li, F. & Ravetch, J.V. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A 109, 6181-6186 (2012)). In a variety of antibody-mediated protection models against bacterial and viral infections, the protective activity of the GASDALIE Fc domain variant of the protective mAb was demonstrated to be significantly enhanced compared to wild-type human IgG1. See Smith, P. et al., Pro Natl Acad Sci US A 109, 6181-6186 (2012); Bournazos, S. et al., Cell 158, 1243-1253 (2014); Bournazos, S. et al., J ClinInvest 124, 725- 729 (2014); and DiLillo, D.J. et al., Nat Med 20, 143-151 (2014).
[0159] More importantly, evaluation of the therapeutic act...
Embodiment 3
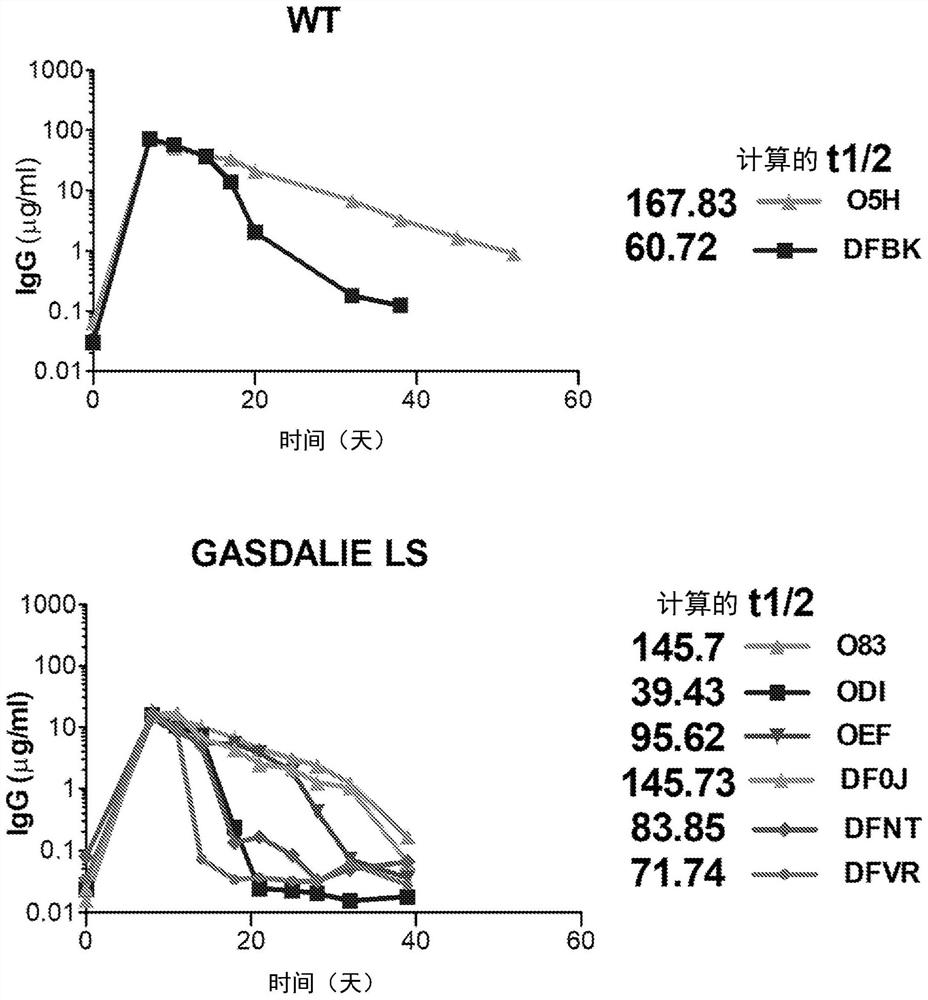
[0164] To further extend the in vivo half-life of the GAALIE variant, it was combined with mutations that increased FcRn affinity without affecting FcγR binding (Zalevsky, J. et al., Nat Biotechnol 28, 157-159 (2010) and Grevys, A. et al., J Immunol. 194, 5497-5508 (2015)). These mutations included M428L and N434S (LS variants, Zalevsky, J. et al., Nat Biotechnol 28, 157-159 (2010)), and the amino acid sequences of the resulting Fc domain variants are shown in Figure 16 middle. Determination of protein melting temperature and binding affinity of FcγR / FcR-enhanced variants to FcRn ( Figure 17-20 ). Furthermore, the in vivo half-lives of these variants were evaluated in FcRn / FcγR humanized mice ( FIG. 21 ). As expected, GAALIE LS (G236A / A330L / I332E / M428L / N434S) showed prolonged half-life, which also translated into prolonged and enhanced Fc effects in the mAb-mediated platelet clearance model in FcγR / FcRn humanized mice child activity ( Figure 22 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com