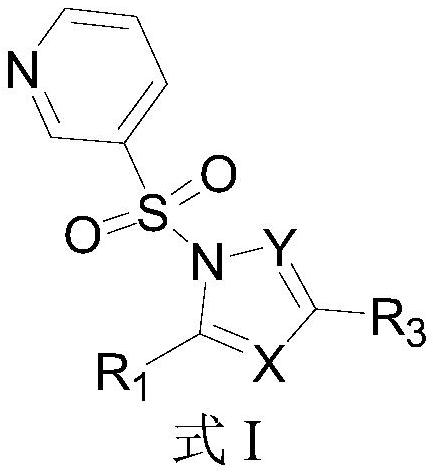
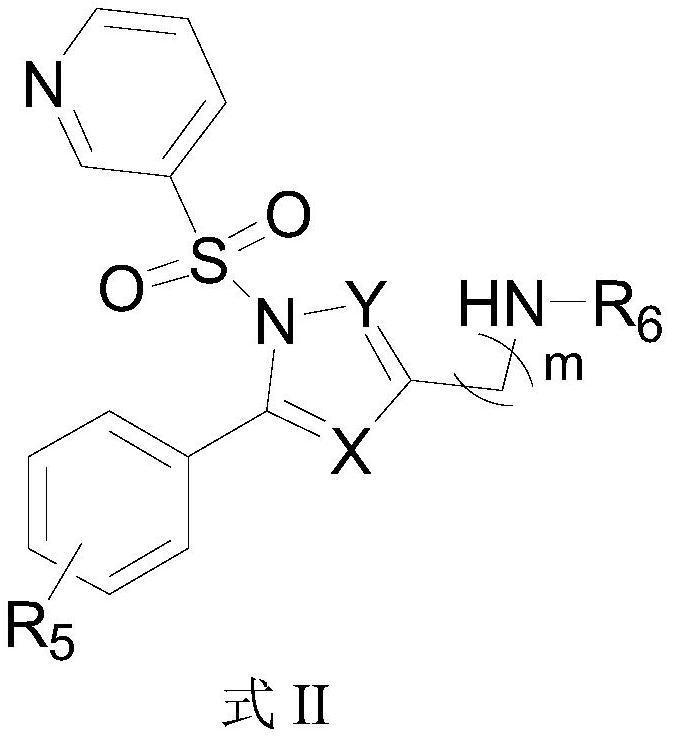
3-pyridinesulfonyl-1-N-heteropyrrole derivatives capable of treating peptic ulcer as well as preparation method and application thereof
A compound and alkoxy technology, applied in the field of 3-pyridinesulfonyl-1-N-heteropyrrole derivatives and their preparation, can solve the problems of easy to cause drug resistance, high recurrence rate of gastric ulcer, high risk and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
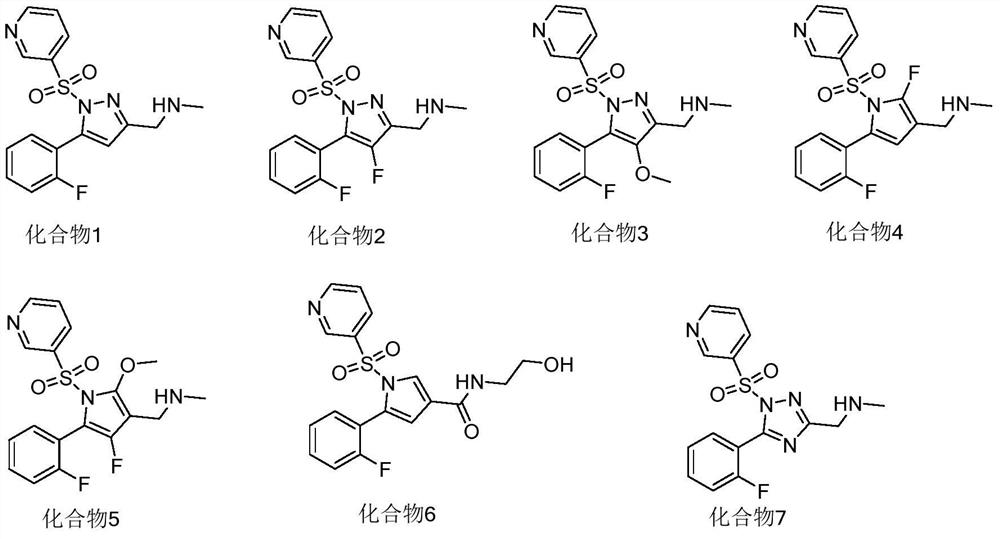
[0083] Embodiment 1, the preparation of compound 1
[0084] The synthetic route is:
[0085]
[0086] The specific preparation method is as follows:
[0087] Add 20.0g (144.78mmol) of compound A, 21.2g (144.78mmol) of diethyl oxalate and 100mL of methanol to the reaction flask, cool the system to 0°C, add dropwise 156.4g of 10% sodium methoxide in methanol, at 20-25 Reaction at ℃ for 4h. Then the pH was adjusted to 2-3 with 5% dilute hydrochloric acid, a large amount of solids precipitated out of the system, the filter cake was rinsed with 100 mL of water and then blast-dried to obtain 22.8 g of compound B with a yield of 75.0%. LC / MS m / z=210.1[M] + .
[0088] Take 20.0g (95.15mmol) of compound B, add 100mL of acetic acid and 3.8g (95.15mmol) of 80% hydrazine hydrate, stir overnight at room temperature, add 500mL of water to produce a large amount of white solid, filter, filter the cake with 50mL DIPEA (N,N' -diisopropylethylamine) was beaten, filtered and dried to obt...
Embodiment 2
[0093] Embodiment 2, the preparation of compound 2
[0094] The synthetic route is:
[0095]
[0096] The specific preparation methods include:
[0097] Take 10.0g (28.9mmol) of compound 1, 10.3g (57.8mmol) of NBS (N-bromosuccinimide) and 100mL of DMF, react at 80°C for 6h, add 300mL of water, a large amount of solid precipitates, suction filter, filter cake Rinse with 50mL of water and air-dry at 70°C to obtain 10.0g of compound H, yield 82.0%, LC / MS m / z=426.1[M+H] + .
[0098] Take 4.5g (10.58mmol) of compound H, 2.9g (31.74mmol) of tetramethylammonium fluoride and 50mL of DMF, react at 80°C for 16h, add 150mL of water, a large amount of solid precipitates, filter with suction, and rinse the filter cake with 50mL of water Washed, air-dried at 70°C to obtain 3.0g of compound 2, yield 78.6%, LC / MS m / z=365.1[M+H] + ; 1HNMR(DMSO-d6):2.43(s,3H),3.87(s,2H),7.03-7.12(m,1H),7.16-7.18(m,2H),7.60-7.63(m,1H),7.74- 7.76(m,1H),7.86-7.90(m,1H),8.56-8.57(m,1H),8.87-8.89(m,1H).
Embodiment 3
[0099] Embodiment 3, the preparation of compound 3
[0100] The synthetic route is:
[0101]
[0102] The specific preparation method is:
[0103] Take 4.0g (9.40mmol) of compound H, add 20mL of methanol, cool down to 0°C, add dropwise 12.2g of 5% methanol solution of sodium methoxide, stir at room temperature for 5h, add 100mL of water, extract twice with 200mL of ethyl acetate, take The organic phase was concentrated under reduced pressure, and the concentrate was purified by a silica gel column (the eluent was a mixed solution of dichloromethane:methanol=5:1) to obtain compound 3 with a yield of 80.2%, LC / MS m / z=377.4 [M +H] + ; 1 HNMR(DMSO-d6):2.43(s,3H),3.87(s,2H),3.89(s,3H),7.03-7.12(m,1H),7.16-7.18(m,2H),7.60-7.63( m,1H),7.74-7.76(m,1H),7.86-7.90(m,1H),8.56-8.57(m,1H),8.87-8.89(m,1H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap