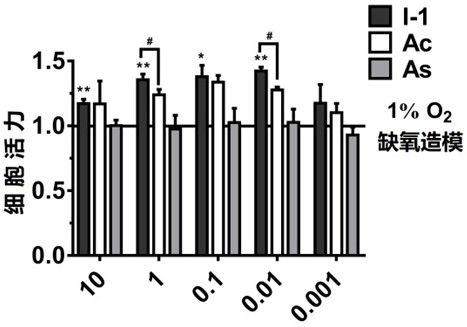
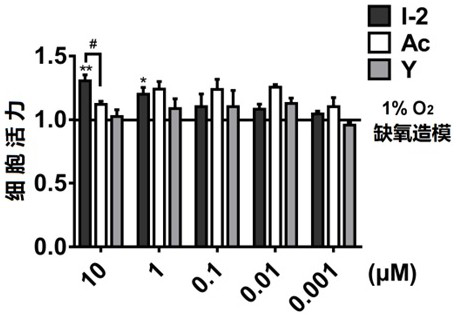
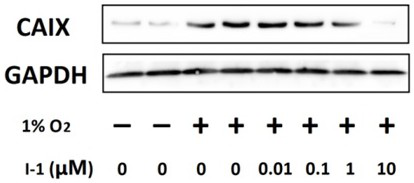
Acetazolamide derivative and its preparation method and application in preparation of medicine for treating coronary heart disease
A technology of acetazolamide and preparation of acetazolamide, applied in the field of preparation of drugs for the treatment of coronary heart disease, can solve problems such as poor therapeutic effect and reduced drug efficacy of myocardial hypoxia, and achieve improved drug efficacy and reduced heart hypoxia area. Decrease and increase the effect of cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. Preparation of 5-amino-1,3,4-thiadiazole-2-sulfonamide (compound 1)
[0046]
[0047] 1
[0048] Weigh 5.0 g of acetazolamide into a flask, add 30 mL of absolute ethanol, stir at room temperature, add 5 mL of concentrated hydrochloric acid, heat the oil bath to reflux at 85 °C, and monitor the reaction by thin-layer chromatography after all the solids are dissolved. After the reaction is complete, spin dry, and adjust the pH to alkaline with a small amount of saturated sodium bicarbonate solution. It was extracted three times with ethyl acetate, and the solvent was spin-dried to obtain 3.5 g of white solid with a yield of 86.4%.
[0049] 1 H NMR (600 MHz, DMSO- d 6 ) δ 8.06 (s, 2H), 7.81 (s, 2H).
Embodiment 2
[0050] Example 2. Preparation of N-(5-sulfonamido-1,3,4-thiadiazol-2-yl)hept-6-yne amide (compound 2)
[0051]
[0052] 2
[0053] Dissolve 277 mg of 6-heptynoic acid in dichloromethane, add 2 equivalents of oxalyl chloride dropwise under ice-cooling, then add a drop of N,N-dimethylformamide, continue stirring for 6-8 h, spin dry for later use. Dissolve 360 mg of compound 1 in N,N-dimethylformamide, add 316 mg of pyridine under ice-cooling, then add dropwise the solution of 6-heptynoyl chloride in N,N-dimethylformamide, and continue stirring for 6 h. The reaction was monitored by thin layer chromatography. After the reaction, the solvent was spin-dried, and 0.42 g of white powder was obtained by silica gel column chromatography, with a yield of 73.7%.
[0054] 1 H NMR (600 MHz, DMSO- d 6 ) δ 13.0 (s, 1H), 8.32 (s, 2H), 2.78 (t, J =2.4 Hz, 1H), 2.55 (t, J = 7.2 Hz, 2H), 2.20–2.17 (m, 2H), 1.73–1.68 (m, 2H),1.50–1.45 (m, 2H).
Embodiment 3
[0055] Example 3. Preparation of 2-bromoethyl 2-acetoxybenzoate (compound 3a)
[0056]
[0057] 3a
[0058] Dissolve 360 mg of aspirin in dichloromethane, add 2 equivalents of oxalyl chloride dropwise in an ice bath, then add a drop of N,N-dimethylformamide, continue stirring for 6-8 h, spin dry for later use. Dissolve 250 mg of 2-bromoethanol in dichloromethane, add 303 mg of triethylamine under ice-cooling, then add a dichloromethane solution of aspirinyl chloride dropwise, continue stirring for 6 h, and monitor the reaction by thin-layer chromatography. After the reaction was finished, it was extracted with dichloromethane, the solvent was spin-dried, and 0.40 g of light yellow oil was obtained by silica gel column chromatography, with a yield of 70.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com