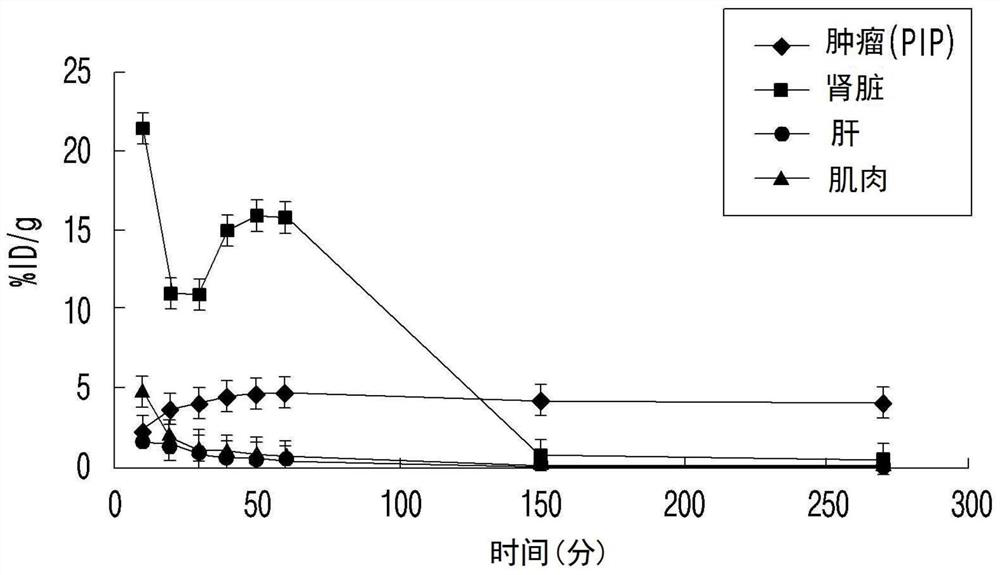
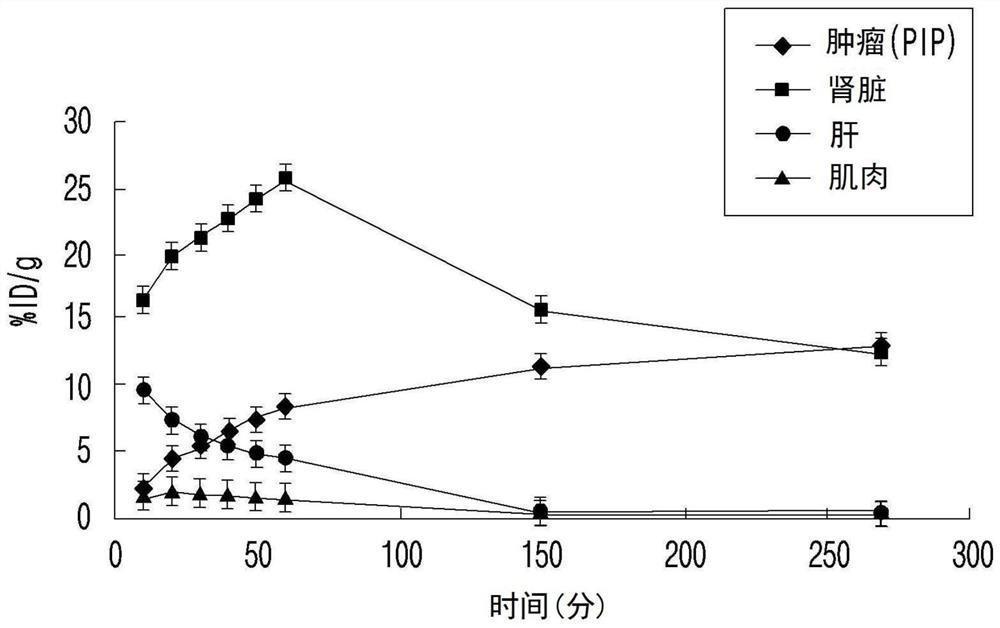
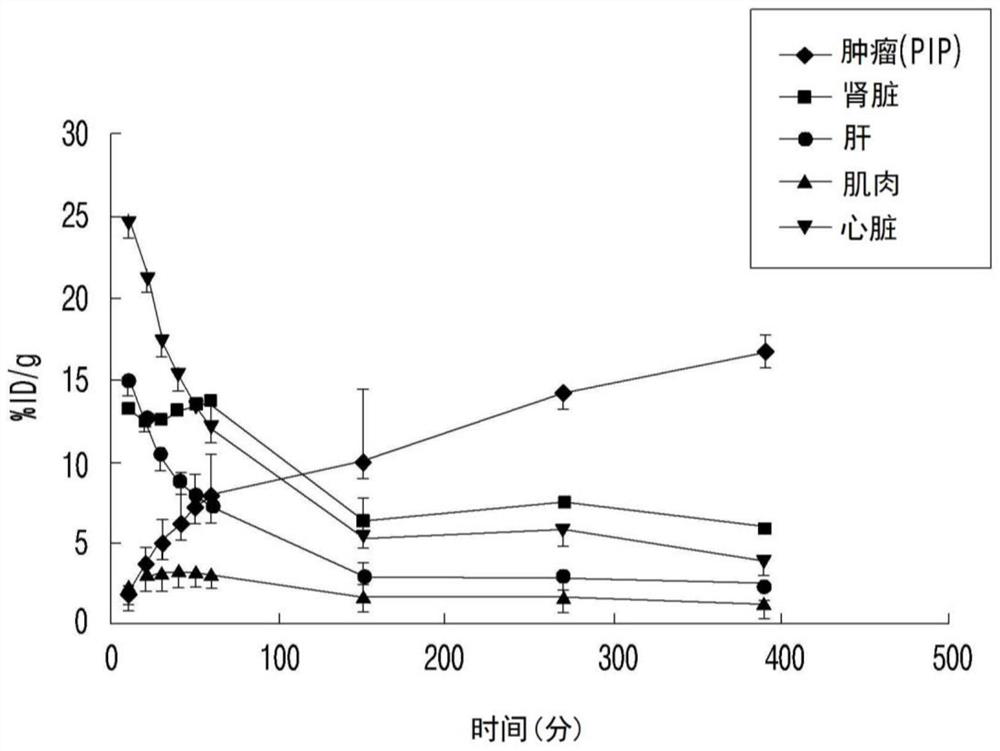
Psma-targeted radiopharmaceutical for diagnosing and treating prostate cancer
A radioactive and pharmaceutical technology, used in radioactive carriers, in vivo radioactive preparations, drug combinations, etc., can solve the problem of prostate cancer having no therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0265] Preparation of Compound 3b, 3c
[0266]
[0267] Preparation of compound 3b
[0268] Compound 3a (5.2 g, 10.66 mmol) was dissolved in dichloromethane (100 mL), cooled to 0 °C, and then tert-butyl bromoacetate (1.9 mL, 12.8 mmol) was added slowly. The mixture was maintained at 0 °C, triethylamine (2.2 mL, 16 mmol) was slowly added, and the temperature was slowly raised to room temperature with stirring. After stirring for 3 hours, water (50 mL) was added and the organic compounds were extracted with dichloromethane (50 mL, twice). The collected organic layer was treated with anhydrous sodium sulfate, concentrated under reduced pressure, and the concentrate was separated by column chromatography (5% methanol / dichloromethane) to obtain compound 3b (3.36 g, 52%).
[0269] 1 H NMR (400MHz, CDCl 3 )δ1.39-1.53(m,36H),1.55-1.89(m,5H),2.02-2.10(m,1H),2.22-2.37(m,2H),2.54-2.58(m,2H),3.27( s,2H),4.28-4.36(m,2H),5.07-5.10(m,2H);
[0270] 13 C NMR (100MHz, CDCl 3 )δ22.6,...
Embodiment 2
[0277] Preparation of Compound 2a, 2b
[0278]
[0279] Step 1: Preparation of Compound 5a
[0280] After dissolving triphosgene (107mg, 0.36mmol) in acetonitrile (5.0mL), compound 3a (500mg, 1.03mmol) dissolved in acetonitrile (10mL) was slowly added thereto at 0°C, and then triethylamine was added (0.50 mL, 3.61 mmol) and stirred for 30 minutes. Added propargylamine (4a, 0.072mL, 1.13mmol) at 0°C, stirred at room temperature for 1 hour after 15 minutes, concentrated under reduced pressure, added water, and repeated extraction of the organic compound with ethyl acetate three times. The collected organic solvent was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography (2% methanol / dichloromethane) to obtain white solid compound 5a (492 mg, 84%).
[0281] 1 H NMR (400MHz, CDCl 3)δ1.25-1.30(m,2H),1.44(s,18H),1.48(s,9H),1.51-1.60(m,3H),1.67-1.76(m,1H),1.80-1.90(m, 1H),2.05-2.13(m,1H),2.18(t,J=2.6Hz,1H),2.29-2.40...
Embodiment 3
[0311] Compound 2c, preparation of 2d
[0312]
[0313] Step 1: Preparation of compound 5c
[0314] Dissolve 4-pentanoic acid (82mg, 0.83mmol) in dichloromethane (10mL), cool to 0°C, then add N,N'-dicyclohexylcarbodiimide (190mg, 0.91mmol) and compound 3c (0.5 g, 0.83 mmol) and stirred at room temperature for 1 hour. After the organic layer was filtered several times, the solvent was removed under reduced pressure, and the concentrate was separated by column chromatography (30% ethyl acetate / n-hexane) to obtain compound 5c (0.29 g, 52%).
[0315] 1 H NMR (400MHz, CDCl 3 )δ1.34-1.67(m,39H),1.68-2.02(m,5H),2.16-2.32(m,2H),2.37-2.56(m,5H),3.22(t,J=7.2Hz,1H) ,3.29(t,J=7.6Hz,1H),3.82-3.90(m,2H),4.17-4.29(m,2H),5.49-5.52(m,1.5H),5.60(d,J=8.0Hz, 0.5Hz);
[0316] MS(ESI) m / z 704[M+Na] +
[0317] Step 1: Preparation of compound 5d
[0318] Except using 4-pentanoic acid (32mg, 0.32mmol), N,N'-dicyclohexylcarbodiimide (74mg, 0.36mmol), compound 3c (0.20g, 0.32mmol), in order...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com