A kind of peony seed meal ace inhibitory peptide and preparation method and application thereof
A technology of peony seed meal and inhibitory peptide, which is applied in peony seed meal ACE inhibitory peptide and its preparation method and application field, can solve the problems of low bioavailability, and achieve the effects of increased release, stable absorption, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
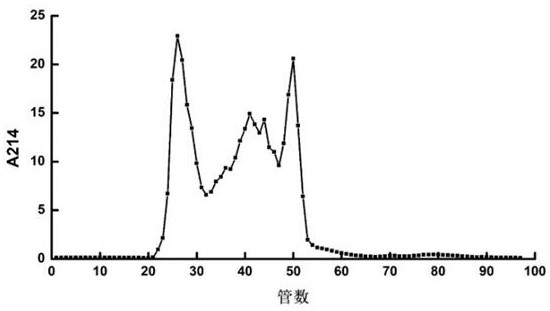
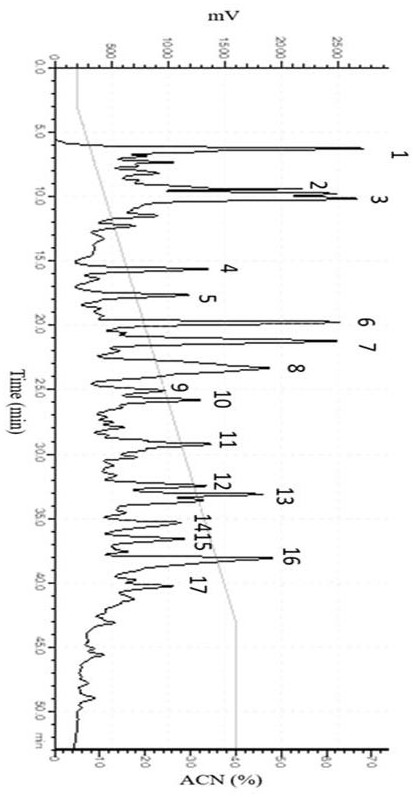
[0025] The separation and purification of the ACE inhibitory peptide of the present invention includes two steps of Sephadex G-25 gel filtration chromatography and reverse phase high performance liquid chromatography (RP-HPLC).
[0026] Preparation of peony seed meal protein enzymatic hydrolyzate: Peony seed meal was crushed, degreased and dried with petroleum ether, and sieved for later use. Using alkali extraction and acid precipitation method, adding NaOH solution to extract protein from degreased peony seed meal, the extraction process conditions are: solid-liquid ratio 1:25 ( w / v ), the extraction pH was 9.25, the extraction temperature was 53°C, and the extraction time was 70 min; the extract was centrifuged at 10,000 r / min for 15 min at 4°C, and the supernatant was taken and the pH was adjusted to 4.0 to precipitate the protein acid. After centrifugation at 10000 r / min for 15 min, the precipitate was collected, redissolved in water, adjusted to neutral pH, and freeze-d...
Embodiment 2
[0032] The activity of the isolated and purified ACE inhibitory peptide was detected. Add 10 μL of 0.1 U / mL ACE enzyme, 50 μL of 1 mmol / L substrate FAPGG, and 40 μL of a certain concentration of sample on a 96-well plate, measure the absorbance value at 340 nm with a microplate reader, and record it as A 1。 Continue to react at 37°C for 30 min, measure the absorbance again, and record it as A 2 , 3 parallel experiments, and the results were averaged. Among them, FAPGG was dissolved in 100 mmol / L boric acid buffer solution (pH 8.3, containing 300 mmol / L NaCl), and deionized water was used as a blank control. ∆A (∆A=A 1 -A 2 ) represents the change of ACE enzyme activity per unit time, and the formula for calculating ACE inhibitory activity is as follows:
[0033]
[0034] In the formula, ∆A s is the change of ACE activity within 30 min when the sample is added, ∆A b It is the change of the absorbance value of the blank group within 30 min.
[0035] From Figure 4 It ...
Embodiment 3
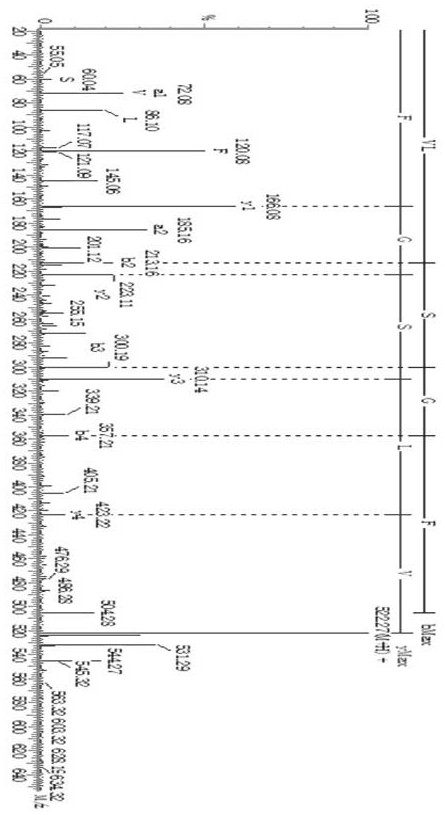
[0037]Molecular docking of ACE inhibitory peptides with ACE. Download the three-dimensional structure file of ACE protein (PDB ID: 1O8A) from the RCSB protein database (http: / / www.rcsb.org / pdb / home / home.do), and use AutoDock Tool 4.0 and AutoDock Vina software to analyze the protein and ACE The preparation and docking of inhibitory peptides, according to the predicted binding energy, select the best binding mode with ACE, and determine the interaction mode between ACE inhibitory peptides and ACE.
[0038] The minimum binding energy between VLSGF and ACE is -8.6 kcal / mol, and the interaction force between VLSGF and ACE includes van der Waals force, hydrogen bonding, and is also related to the interaction of metal ions. From Figure 5 It can be seen that VLSGF not only forms hydrogen bonds with the three amino acids Ala354, Glu384 and Tyr523 of the active pocket S1 of the ACE protein molecule, but also forms hydrogen bonds with His353 and His513 of the active site pocket S2, in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elution gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com