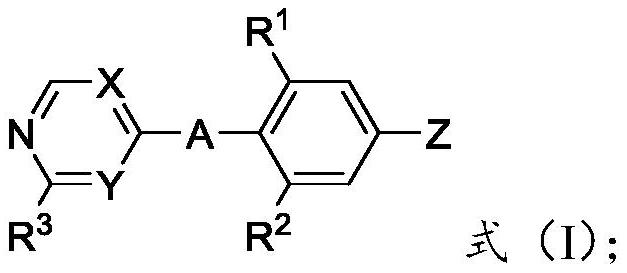
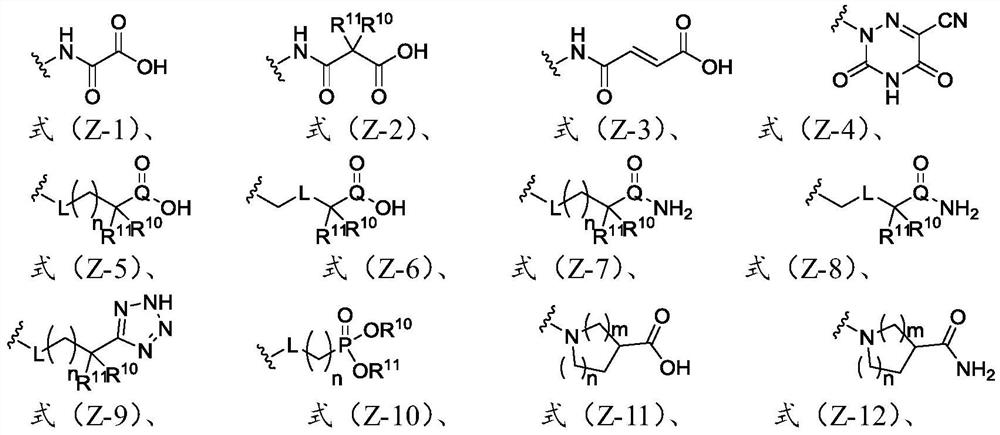
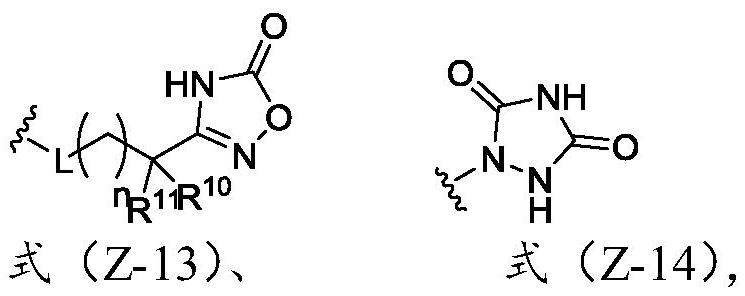
Heterocyclic compound and application thereof
A technology of heterocyclic compounds and heteroaryl groups, applied in the field of medicinal chemistry, can solve problems such as poor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention has no special requirements on the preparation method of the aforementioned heterocyclic compound, and those skilled in the art can select a suitable preparation process based on the target product based on the general knowledge of synthesis. Can be synthesized as follows:
[0050]
[0051] Including but not limited to the following specific preparation process:
[0052]
[0053] a. Alkali (potassium carbonate, cesium carbonate, sodium carbonate, etc.), cuprous iodide, N,N-dimethylformamide
[0054] b. Ethyl acrylate, trifluoroacetic acid c. Sodium hydroxide, water, methanol
[0055] d. Ethyl chloroformylacetate, N,N-diisopropylethylamine, tetrahydrofuran e. Sodium hydroxide, water, methanol
[0056] f. Hydrochloric acid, sodium nitrite, N-cyanoacetylurethane, water, pyridine g. Acetic acid, sodium acetate.
[0057] The present invention also provides an application of the heterocyclic compound described in the present invention in the pre...
Embodiment 1
[0073] 2-(3,5-Dichloro-4-((7-methyl-7H-pyrrole[2,3-d]pyrimidin-4-yl)oxy)-phenyl)-3,5-dioxo -2,3,4,5-Tetrahydro-[1,2,4]triazine-6-carbonitrile
[0074]
[0075] Step 1: Preparation of 2,6-dichloro-4-aminophenol
[0076] 2,6-Dichloro-4-nitrophenol (4.2g, 0.02mol) was dissolved in ethanol (20ml) and water (4ml), and acetic acid (2ml) was added. Then heat and stir to raise the temperature to 90° C., and add Fe powder (5.6 g, 0.1 mmol) in batches. After the addition, the reaction solution was kept warm for 30 minutes, then the heating was stopped, and it was filtered while it was hot. The product was finally separated by column chromatography (ethyl acetate / petroleum ether=1 / 3). 2,6-Dichloro-4aminophenol (3.35 g, 94.1% yield) was obtained.
[0077] LC-MS (m / z) 177.2 (M-1).
[0078] Step 2: Preparation of 4-chloro-7-methyl--7H-pyrrolo[2,3-d]pyrimidine
[0079] Dissolve 4-chloro-7H-pyrrole[2,3-d]pyrimidine (615mg, 4mmol) in tetrahydrofuran (10ml), under nitrogen protection, ...
Embodiment 2
[0092] 3-((3,5-dichloro-4-((7-methyl-7H-pyrrole[2,3-d]pyrimidin-4-yl)oxy)-phenyl)-amino)-3-oxo propionic acid
[0093]
[0094]Step 1: Preparation of 3-((3,5-dichloro-4-((7-methyl-7H-pyrrole[2,3-d]pyrimidin-4-yl)oxy)-phenyl)-amino) -Ethyl 3-oxopropionate
[0095] 3,5-Dichloro-4-((7-methyl-7H-pyrrole[2,3-d]pyrimidin-4-yl)oxy)aniline (62mg, 0.2mmol) was dissolved in tetrahydrofuran (3ml) , and N,N-diisopropylethylamine (65 mg, 0.5 mmol) was added. Then the temperature was lowered to 0-5°C, and ethyl chloroformoacetate (45mg, 0.3mmol) was added. After the addition was complete, the mixture was raised to room temperature and stirred for 2 hours. After the reaction of the raw materials was complete, water was added, followed by ethyl acetate for extraction, the combined organic phases were dried, filtered, and evaporated under reduced pressure to obtain a residue, which was finally separated by column chromatography (ethyl acetate / petroleum ether=1 / 2). 3-((3,5-Dichloro-4-((...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap