Application of curcumenol derivative in preparation of antitumor drugs
A technology of anti-tumor drugs and derivatives, which is applied in the field of medicine and can solve the problems that there are no reports on the anti-tumor activity of curcumol derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: in vitro experiment
[0018] 1. Materials and methods
[0019] 1. Test material
[0020] Glioma U251 cells were purchased from ATCC and cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin at 37°C, 5% CO 2 Cultivate in an incubator and pass passage for 2-3 days.
[0021] Fetal bovine serum and RPMI 1640 medium were purchased from Gibco, USA.
[0022] Curcumol was purchased from Shanghai Baoman Biotechnology Co., Ltd., with an HPLC purity of ≥98%.
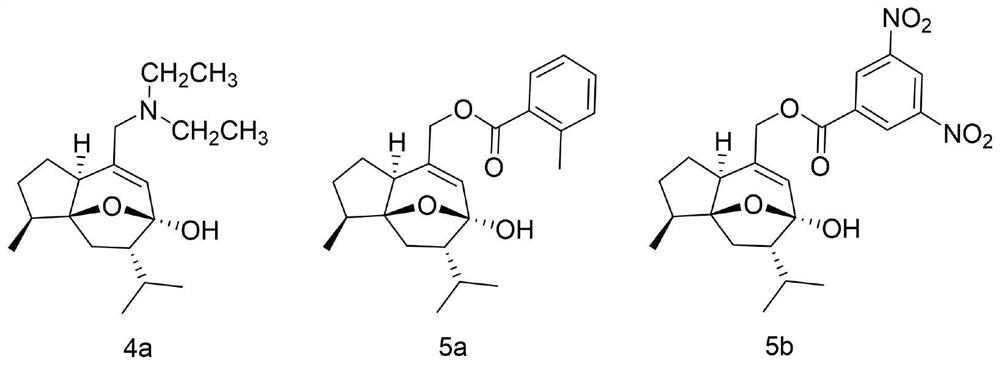
[0023] The chemical structures of curcumol derivatives 4a, 5a and 5b are as figure 1 As shown, it was synthesized according to the literature method, and the purity was not less than 98%.
[0024] 2. MTT method to determine the IC50 value of the compound on the inhibition of the proliferation of glioma U251 cells
[0025] Take the glioma U251 cells in the logarithmic growth phase, and use 2.5×10 per well 4 Cells were cultured in a 96-well plate at...
Embodiment 2
[0033] Embodiment 2: experiment in vivo
[0034] 1. Materials and methods
[0035] 1. Test material
[0036] Glioma U251 cells were purchased from ATCC and cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U / ml penicillin, and 100 μg / ml streptomycin at 37°C, 5% CO 2 Cultivate in an incubator and pass passage for 2-3 days.
[0037] Fetal bovine serum and RPMI 1640 medium were purchased from Gibco, USA.
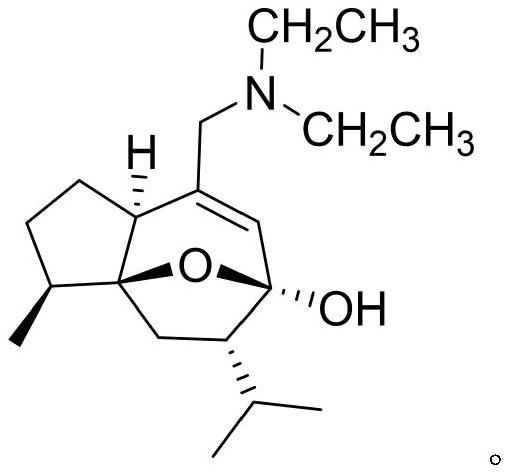
[0038] The chemical structure of curcumol derivative 4a is as figure 1 As shown, it was synthesized according to the literature method, and the purity was not less than 98%.
[0039] Male BALB / c nude mice aged 4-6 weeks, with a body weight of (20±2) g, were raised under SPF conditions for at least one week.
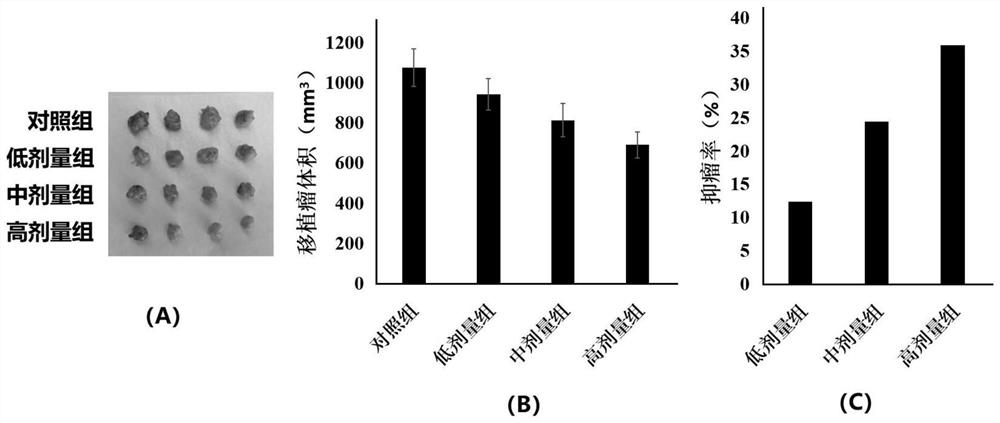
[0040] 2. Tumor transplantation test in nude mice
[0041] BALB / c nude mice were randomly divided into 4 groups: control group, derivative 4a low dose group, derivative 4a medium dose group and derivative 4a high dose group, 4 mice in each group. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com