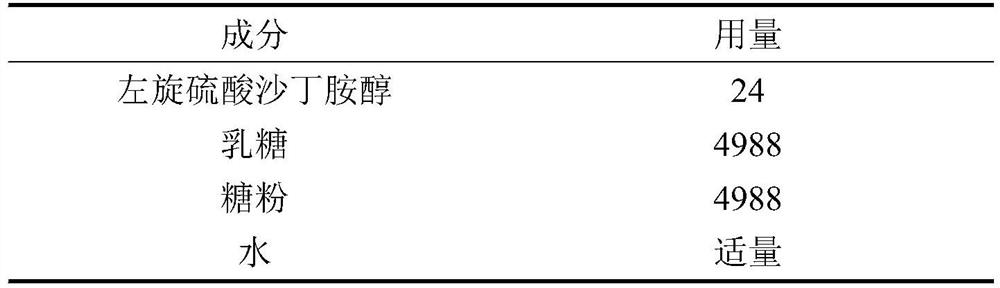
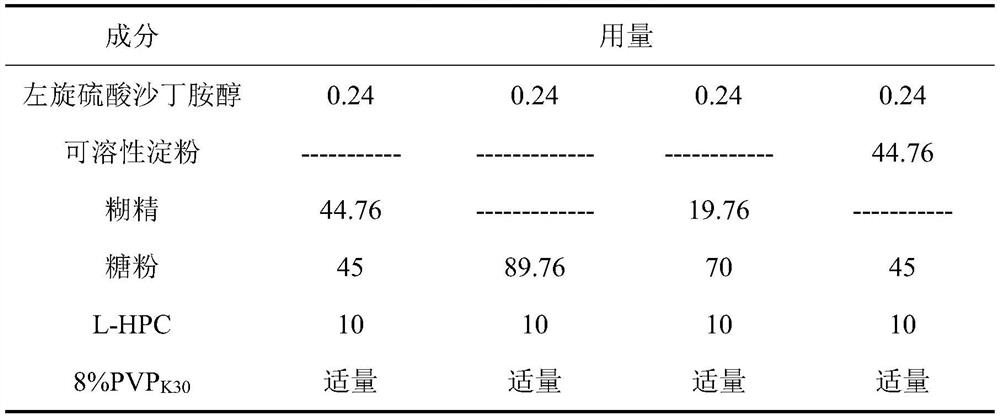
Preferred method for treating respiratory diseases by using L-salbutamol sulfate
A kind of albuterol and disease technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Example 1: Clinical application of L-sulphate albuterol granules and tablets in the treatment of bronchial asthma and asthmatic bronchitis
[0022] Take random, double-blind, parallel control, multi-center clinical study, tablet enrolls 197 cases altogether, wherein test group (L-salbutamol sulfate tablet) 99 cases, control group (B group) 98 cases. L-salbutamol sulfate tablet 2mg is used for the treatment of bronchial asthma and chronic asthmatic bronchitis Compared with commercially available racemic salbutamol sulfate tablet 4mg, its dosage is 1 / 2 of racemic salbutamol sulfate tablet. Observe the clinical control rate, total effective rate and incidence of adverse reactions of patients after administration.
[0023] A randomized, open, parallel controlled, multi-center clinical study was adopted. A total of 99 cases of L-salbutamol sulfate granules (Group C) were enrolled, and its dosage of 2 mg was 1 / 2 of that of racemic salbutamol sulfate tablets. Observe the clin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com