Preparation method of compound bone peptide injection
A compound osteopeptide and injection technology, applied in the field of medicine, can solve the problems of low extraction efficiency of bone peptide content, long-term storage clarity decrease, long-term storage unresolved clarity, etc., to achieve the effect of enhancing the overall stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
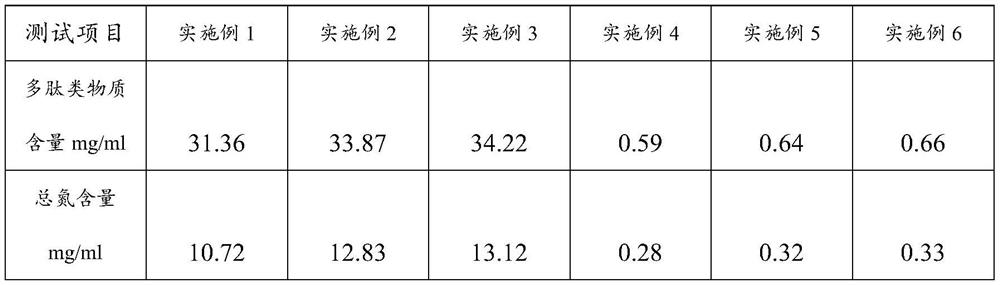
[0034] Embodiment 1, the preparation of bone peptide extract
[0035] The preparation method of described bone peptide extract is:
[0036] 1) Grind pig limb bones, add 10 times the mass of water, and heat at 105°C and 1kg / cm 2 Extract 2 times under pressure conditions, 1 hour each time, combine the extracts extracted twice and filter;
[0037] 2) the filtrate is left to cool, add petroleum ether extraction of 1 / 5 of the filtrate quality, remove the upper layer of grease, concentrate to 1 / 7 of the original volume, add ethanol to the concentrated solution, so that the ethanol volume content in the filtrate is 65%, statically Set aside for 18h, filter, and distill the filtrate to 1 / 3 of the original volume;
[0038] 3) Use hydrochloric acid to adjust the pH of the filtrate obtained in step 2) to 5, remove the acid protein, filter, use sodium hydroxide to adjust the pH to 7 again, remove the alkali protein, filter with a 0.2 μm filter element, and then use an ultrafiltration me...
Embodiment 2
[0039] Embodiment 2, the preparation of bone peptide extract
[0040] The preparation method of described bone peptide extract is:
[0041] 1) Crush the bones of pig limbs, add 10 times the mass of water at 120°C and 2kg / cm 2 Under pressure conditions, extract 2 times, 2 hours each time, combine the extracts extracted twice and filter;
[0042] 2) The filtrate is allowed to stand for cooling, adding 1 / 5 of the quality of the filtrate to extract with petroleum ether, removing the upper layer of grease, concentrating to 1 / 6 of the original volume, adding ethanol to the concentrated solution so that the ethanol volume content in the filtrate is 75%, statically Set aside for 20h, filter, and distill the filtrate to 1 / 5 of the original volume;
[0043] 3) Use hydrochloric acid to adjust the pH of the filtrate obtained in step 2) to 3, remove the acid protein, filter, use sodium hydroxide acid to adjust the pH to 9 again, remove the alkali protein, filter with a 0.2 μm filter elem...
Embodiment 3
[0044] Embodiment 3, the preparation of bone peptide extract
[0045] The preparation method of described bone peptide extract is:
[0046] 1) Crush the bones of pig limbs, add 10 times the mass of water at 110°C and 2kg / cm 2 Under pressure conditions, extract 2 times, 2 hours each time, combine the extracts extracted twice and filter;
[0047] 2) The filtrate is allowed to stand for cooling, adding 1 / 5 of the quality of the filtrate to extract with petroleum ether, removing the upper layer of grease, concentrating to 1 / 6 of the original volume, adding ethanol to the concentrated solution so that the ethanol volume content in the filtrate is 75%, statically Set aside for 18h, filter, and distill the filtrate to 1 / 4 of the original volume;
[0048] 3) Use hydrochloric acid to adjust the pH of the filtrate obtained in step 2) to 4, remove the acid protein, filter, use sodium hydroxide to adjust the pH to 8 again, remove the alkali protein, filter with a 0.2 μm filter element, ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap