Methods and compositions for drugs to treat ophthalmic diseases
A technology of pharmacy and compounds, applied in metabolic diseases, drug combinations, sensory diseases, etc., can solve the problems of no phenotype and no immunodeficiency in mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
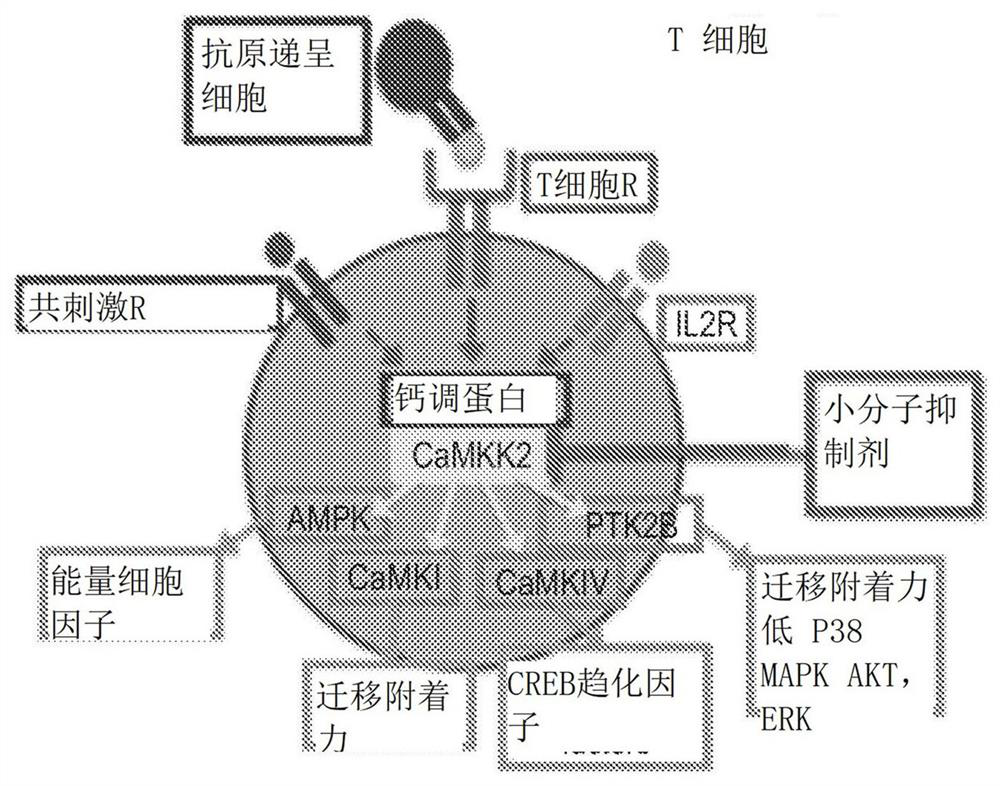
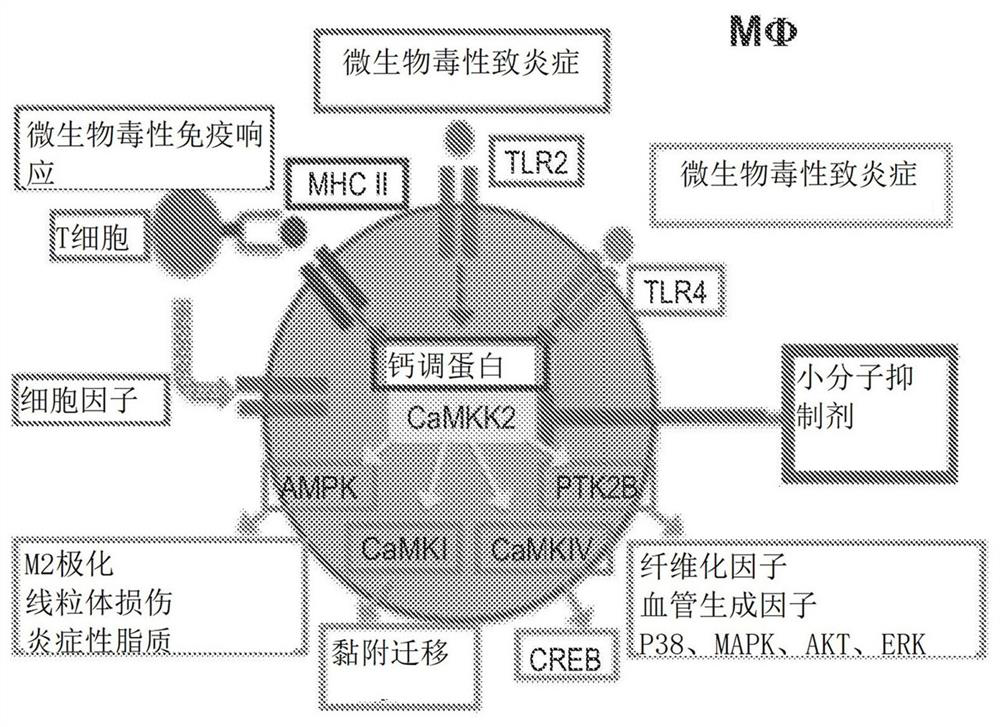
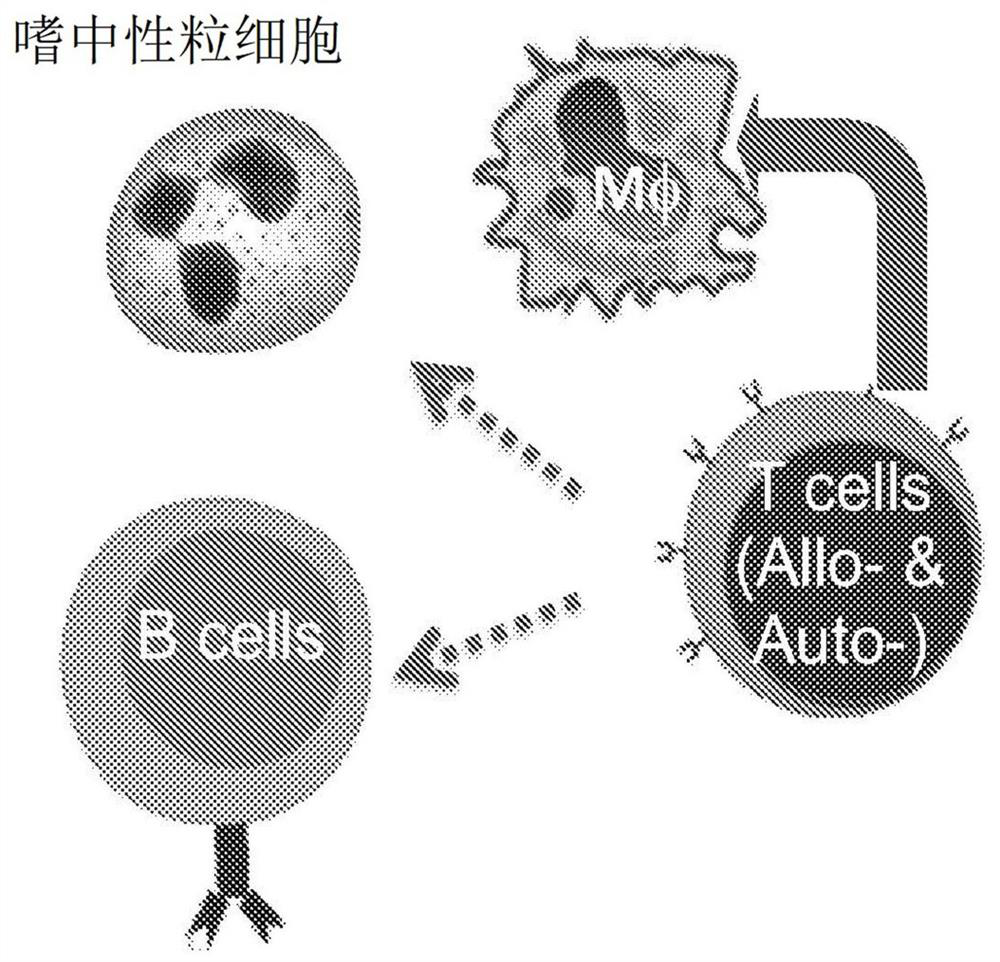
Image
Examples
example 1
[0123] Development of novel small molecule inhibitor compounds
[0124] Computational modeling of the crystal structure of STO-609 / CaMKK2 was used to develop small molecule inhibitors of CaMMK2 (SMIC) based on computational chemistry. In particular, the crystal structure of STO-609 / CaMKK2 was obtained, modifying part of the tool compound, which was not expected to interfere with receptor binding, but should have improved solubility (calculated LogP ~ 3), whereas cell penetration No adverse effects (total polar surface area < 140 considered favorable). Five (5) starting compounds were synthesized by MonomerChem (RTP, NC) and used to generate the data in this paper.
[0125] Table 1 below illustrates the five compounds initially synthesized, each representing a new subclass.
[0126] Table 1
[0127] Compound 1-10
[0128]
[0129]
[0130]
example 2
[0132] Biochemical activity against CaMKK2
[0133] Initial screening of compounds EY301-EY305 was performed in vitro in HEK293 cells (both tested at 10 μΜ). Briefly, HEK293 cells were cultured to subconfluence in RPMI-40 complete medium and 1% fetal bovine serum (FBS) at 37 °C in 5% CO 2 incubate. Cultured cells were then switched to serum-free medium overnight before starting the experiment. Cells were then pretreated with one of five SMICs (EY301, EY302, EY303, EY304, and EY305, all at a concentration of 10 μM), STO-609 (10 μM), or vehicle control at 37°C and incubated at 5 %CO 2 Incubate for 2 hours. Cells were then stimulated with the calcium ionophore ionomycin (0.5 mg / mL) for 15 min and then washed, harvested and lysed to recover total protein using standard methods. After standard protein gel electrophoresis, phospho-CaMKK2 ( Figure 4A ) or phospho-AMPK ( Figure 4B ) for western blotting. Studies were performed in triplicate. Densitometry analysis revealed t...
example 3
[0135] Functional screening of compounds 1-5
[0136] One-way mixed lymphocyte reaction (MLR) ( Figure 5 ) will be used to screen the functional inhibition ability of EY301-EY305. Spleen cells from C57BL / 6 mice were used as responder cells, and spleen cells from BALB / c mice (irradiated with Co60, 3000 rads) were used as stimulator cells. The two types of cells were then mixed in the same volume and concentration and incubated at 37°C in low serum conditions in 5% CO 2Incubate for 24 hours. Methods were 1) vehicle control; 2-6) one of five SMICs (EY301-EY305), or 7) STO-609 (concentrations of 10 μM, 3 μM, 1 μM, 0.3 μM and 0.1 μM). Each concentration / condition will be replicated three times. The supernatant was then recovered and concentrated for analysis by enzyme-linked immunosorbent assay (ELISA) for several major T-cell and macrophage-derived cytokines and effector molecules that are thought to pass through the infiltrated immune system. cells to mediate destructive i...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap