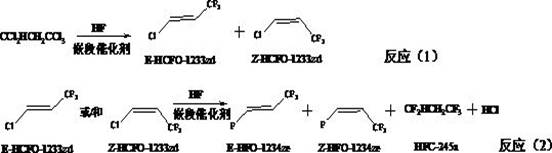
The preparation method of e-1-halo-3,3,3-trifluoropropene
A technology of trifluoropropene and Z-1-, which is applied in the direction of preparation of halogenated hydrocarbons, dehalogenation preparation, halogen substitution preparation, etc., can solve the problems of low selectivity, high reaction pressure, difficult to recycle, etc., and achieve high activity , high yield per pass and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
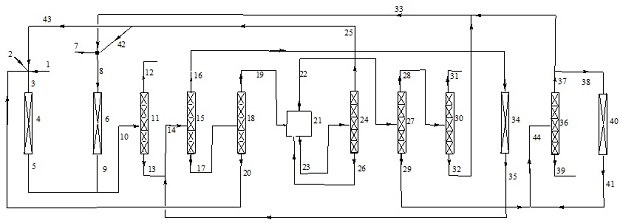
Image
Examples
Embodiment 1
[0054] Preparation of block catalyst: (1) Dissolve tungsten trichloride in water, then dropwise add 10% by mass ammonia water to completely precipitate metal ions, adjust the pH value to 7.0-9.0, and make it fully precipitate under stirring conditions , aged for 24 hours, filtered the formed slurry, then dried at 150°C for 18 hours, pulverized the solid, and pressed into shape to obtain a carrier precursor; the obtained carrier precursor was roasted at 400°C under a nitrogen atmosphere After 18 hours, it was activated at 300°C for 18 hours with a mixed gas consisting of hydrogen fluoride and nitrogen with a molar ratio of 1:2 to obtain a carrier, which was confirmed to be tungsten oxyfluoride by XPS detection; (2) in dry, high-purity In nitrogen or helium or argon atmosphere, according to 20% SbF in block catalyst 5 Composed of 80% tungsten oxyfluoride mass percentage content, the precursor of the active component SbCl 5 Coating on tungsten oxyfluoride to obtain a catalyst pr...
Embodiment 2
[0057] The same operation as in Example 1, the difference is that the block catalyst is made of 30%SbF 5 Composed with 70% tungsten oxyfluoride, and the reaction temperature was changed to 100°C, the reaction result was: the conversion rate of 1,1,1,3,3-pentachloropropane was 54.6%, E-1-chloro-3,3, The selectivity of 3-trifluoropropene was 99.1%, and the selectivity of Z-1-chloro-3,3,3-trifluoropropene was 0.9%.
Embodiment 3
[0059] The same operation as in Example 1, the difference is that the block catalyst is made of 20%SbF 5 Composed of 80% tungsten oxyfluoride, and the reaction temperature was changed to 150°C, the reaction result was: the conversion rate of 1,1,1,3,3-pentachloropropane was 71.6%, E-1-chloro-3,3, The selectivity of 3-trifluoropropene was 97.4%, and the selectivity of Z-1-chloro-3,3,3-trifluoropropene was 2.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com