Method for preparing 3,3,3-trifluoropropyne by gas-phase dehydrohalogenation
A technology for dehydrohalogenation and trifluoropropyne, applied in the fields of dehydrohalogenation preparation, chemical instruments and methods, catalyst activation/preparation, etc. The effect of high efficiency and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
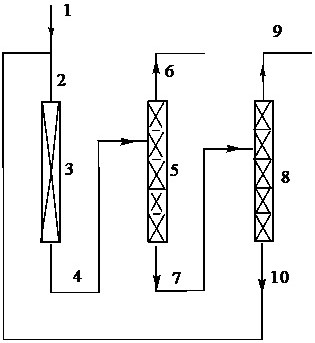
[0051]Catalyst preparation: (1) Dissolve barium chloride in water, then add ammonia water dropwise until the pH value is 7-9, then age for 16 hours, filter, wash, and dry at 100°C for 16 hours to obtain a solid, pulverize, Press molding to obtain the precursor barium hydroxide; (2) The obtained precursor is roasted at 400°C for 12 hours in a nitrogen atmosphere; Gas activation for 16 hours to obtain barium fluoride; (3) according to the mass percentage of cesium fluoride and barium fluoride composition 10%:90%, the alkali metal fluoride is dissolved in water, and the barium fluoride carrier is added to the impregnating solution , impregnated at room temperature for 12 hours, filtered, dried at 100° C. for 16 hours, and calcined at 400° C. for 12 hours under a nitrogen atmosphere to obtain a catalyst.
[0052] A tubular reactor made of Inconium alloy with an inner diameter of 1 / 2 inch and a length of 30 cm was filled with 10 ml of the catalyst prepared above. The temperature o...
Embodiment 2
[0054] The same operation as in Example 1, the difference is that "composition of 10% according to the mass percentage of cesium fluoride and barium fluoride: 90%" is changed to "composition of 20% according to the mass percentage of cesium fluoride and barium fluoride: 80% %", change the reaction temperature to 350°C. The reaction results are as follows: the conversion rate of Z-1-chloro-3,3,3-trifluoropropene is 13.8%, the selectivity of 3,3,3-trifluoropropyne is 96.8%, and the selectivity of E-1-chloro-3 , The sum of the selectivities of 3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene was 3.1%.
Embodiment 3
[0056] The same operation as in Example 1, except that "composition of 10% according to the mass percentage of cesium fluoride and barium fluoride: 90%" was changed to "composition of 30% according to the mass percentage of cesium fluoride and barium fluoride: 70% %", change the reaction temperature to 450°C. The reaction results are as follows: the conversion rate of Z-1-chloro-3,3,3-trifluoropropene is 25.4%, the selectivity of 3,3,3-trifluoropropyne is 93.1%, and the selectivity of E-1-chloro-3 , The sum of the selectivities of 3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene was 6.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com