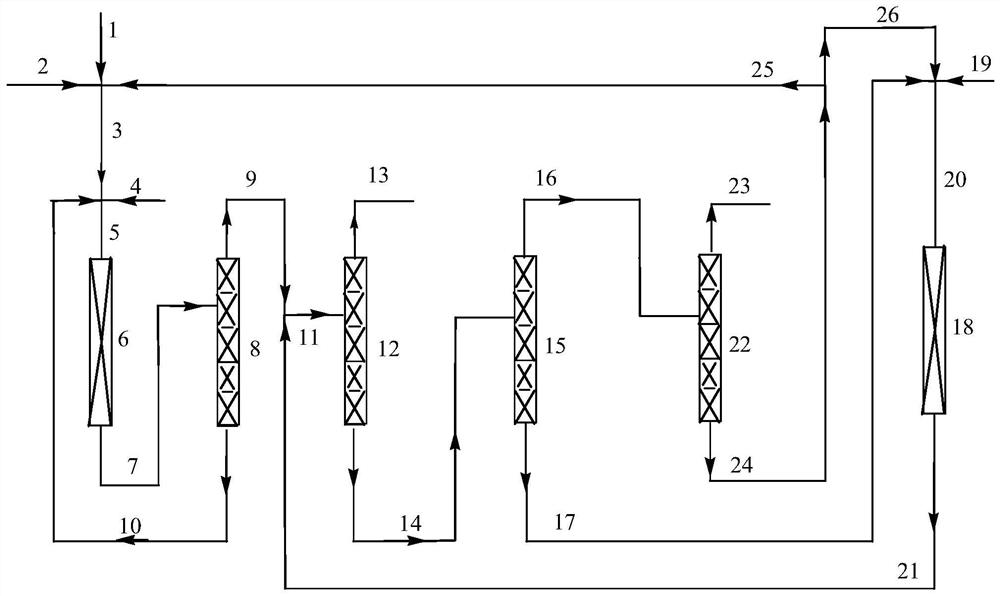
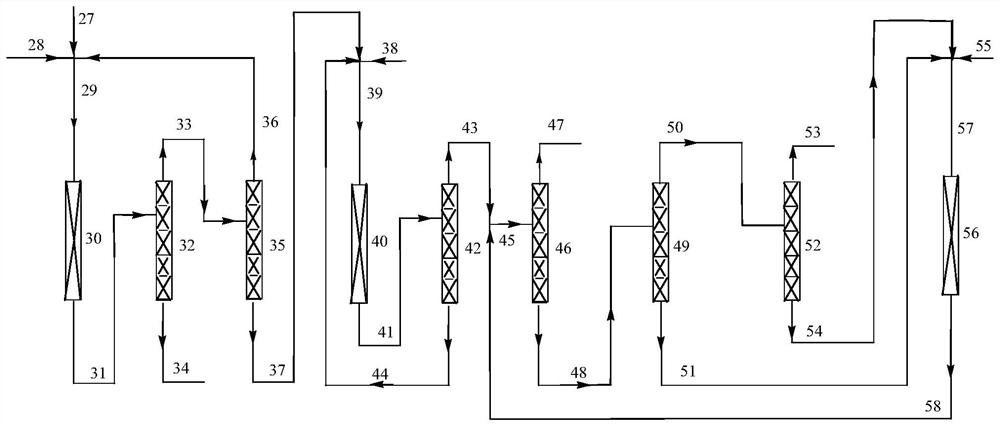
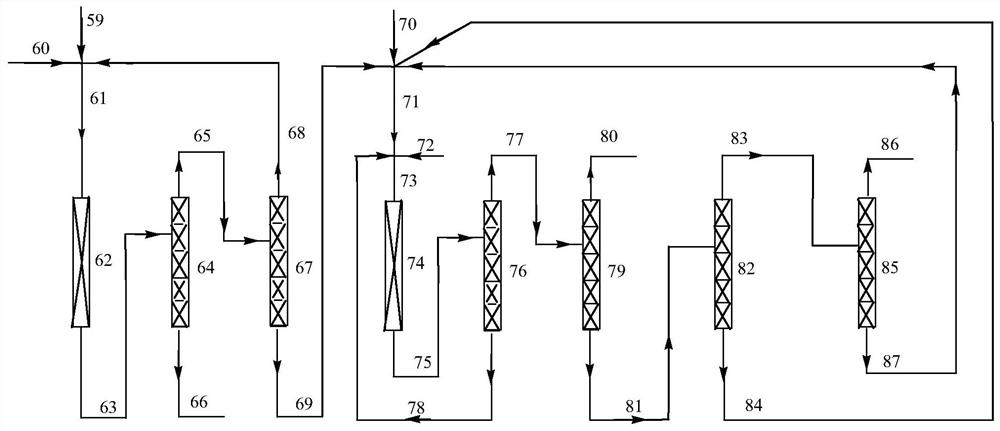
Method for synthesizing 2, 3, 3, 3-tetrafluoro-2-(trifluoromethyl)propionitrile through gas-phase catalytic fluorination
A technology of trifluoromethyl and methyl propionitrile is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Environmental pollution and other problems, to achieve the effect of short synthesis route and high single-pass yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation of fluorine-halogen exchange catalyst: according to the mass percentage content of chromium ions (trivalent or / and tetravalent or / and pentavalent chromium ions) and cobalt elements are 90% and 10%, chromium chloride and chlorinated Cobalt is dissolved in water, and the precipitant ammonia water is added at 60°C, and the pH of the solution is controlled between 7 and 9, so that it is fully precipitated under stirring conditions, and then aged for 10-24 hours, the formed slurry is filtered, and deionized water is used. Wash to neutrality, then dry at 150°C for 10-24 hours to obtain a solid, pulverize the above-mentioned solid, press and shape to obtain a catalyst precursor, and then calcinate the catalyst precursor at 450°C for 10-24 hours under a nitrogen atmosphere. Activation at 300°C with a mixture of hydrogen fluoride and nitrogen with a molar ratio of 1:2 for 10-24 hours, and oxidation at 300°C with a mixture of chlorine trifluoride and nitrogen with a mo...
Embodiment 2
[0064] The same operation as in Example 1, the difference is that "according to the mass percentage content of chromium ions (trivalent or / and tetravalent or / and pentavalent chromium ions) and cobalt elements are 90% and 10%, the chlorine Chromium chloride and cobalt chloride are dissolved in water” to “dissolve chromium chloride and magnesium chloride in water according to the mass percentage of chromium ions and magnesium elements of 90% and 10%”. The reaction results are as follows: the conversion of isobutyronitrile is 100%, the selectivity of 2-chloro-3,3,3-trifluoro-2-(trifluoromethyl)propionitrile is 59.5%, the selectivity of 2-chloro-3, 22.0% selectivity for 3,3-trifluoro-2-(monochlorodifluoromethyl)propionitrile, 2-chloro-3,3,3-trifluoro-2-(trichloromethyl)propionitrile The selectivity was 12.1% and the selectivity for 2,3,3,3-tetrafluoro-2-(trifluoromethyl)propionitrile was 6.4%.
Embodiment 3
[0066] The same operation as in Example 1, the difference is that "according to the mass percentage content of chromium ions (trivalent or / and tetravalent or / and pentavalent chromium ions) and cobalt elements are 90% and 10%, the chlorine Chromium and cobalt chloride are dissolved in water" to "according to the mass percentage content of chromium ions (trivalent or / and tetravalent or / and pentavalent chromium ions) and zinc elements are 90% and 10%, the chlorine Chromium and zinc chloride dissolve in water". The reaction results are as follows: the conversion of isobutyronitrile is 100%, the selectivity of 2-chloro-3,3,3-trifluoro-2-(trifluoromethyl)propionitrile is 62.1%, the selectivity of 2-chloro-3, The selectivity of 3,3-trifluoro-2-(monochlorodifluoromethyl)propionitrile was 28.7%, and the selectivity of 2-chloro-3,3,3-trifluoro-2-(trichloromethyl)propionitrile The selectivity was 8.7% and the selectivity for 2,3,3,3-tetrafluoro-2-(trifluoromethyl)propionitrile was 0.5%....
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com