Single molecule sequencing peptides bound to the major histocompatibility complex
A technology of histocompatibility and complex, which is applied in the fields of protein and peptide sequencing and peptide identification, and can solve problems such as questioning the validity of the sequence list
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0125]Example 1 - Peptide bonded to MHC using identification information and quantity obtained by sequencing
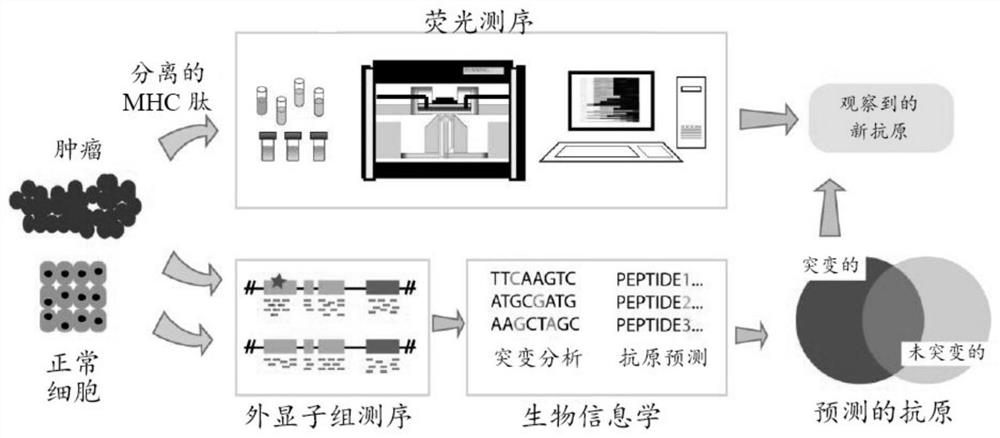
[0126]Method for analyzing MHC peptidesfigure 2 in. In general, the method is divided into four parts: (a) for extracting and enriches the MHC binding peptide from a biological sample, (b) labeled amino acids with fluorophore and perform fluorescence sequencing data, (c) the organism Samples were subjected to genome and transcription group sequencing, and (d) integrated fluorescence sequencing data and genome data to biological information analysis to obtain a list of potential MHC peptide sequences. These examples are each elaborated in more detail below.
[0127]A. Extract MHC binding peptide:
[0128]A variety of methods for enriching and extracting MHC binding peptides have been well described in the literature (Yadav et al., 2014; Müller et al., 2006). The cells and tissues were first dissolved, and MHC protein was enriched by immunoprecipitation methods. Briefly, MHC-I allele-...
example 2
[0140]Example 2 - Computer simulated fluorescence sequencing to verify its application in MHC peptide
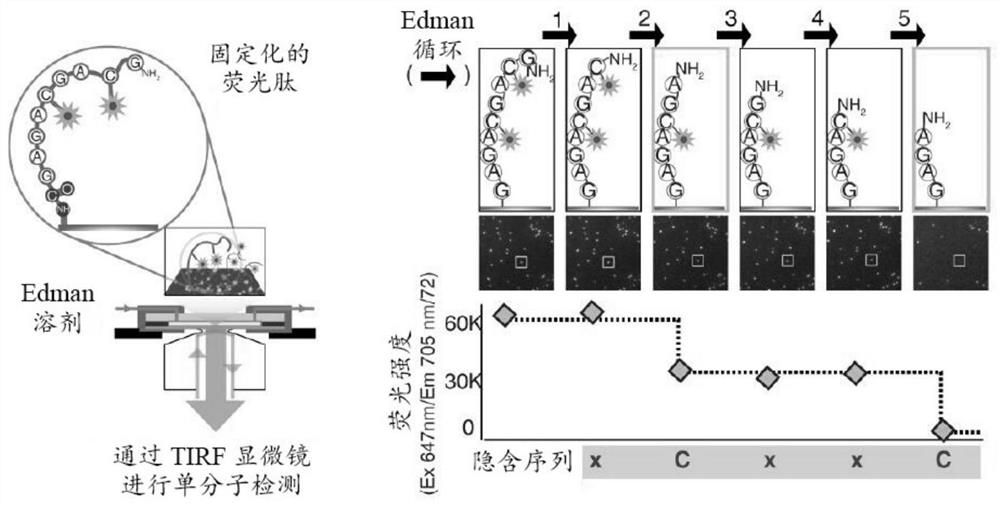
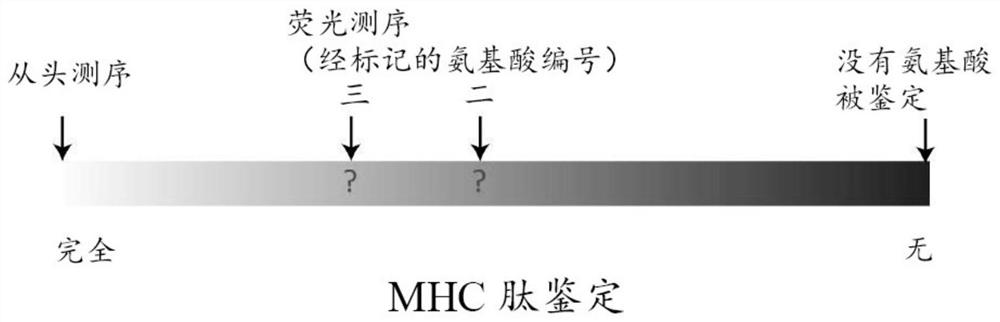
[0141]The MHC peptide is performed in fluorescence sequencing to identify the information content of the sequence between the two extremes, such asimage 3 Simply shown in the simply shown. At one end of the scale, when there is no anhydric acid being labeled, there is no information of the MHC peptide. At the other end of the scale, all amino acid identification information is known, and the MHC peptide can be fully identified. Part of the amino acid labeling scheme performed by fluorescence sequencing is located in the middle of this information scale. In order to determine the location of the information derived from the fluorescence sequencing, different marking methods are simulated to determine the markup strategy for maximizing the information content and verify the application of the MHC peptide profile tool.
[0142]The following two simulation studies emphasize the feasibility ...
example 3
[0148]Example 3 - Sequencing HLA peptide
[0149](i) HLA peptide from single allele B cells
[0150]A pilot experiment is provided in a single allele B cell line to obtain and verify the HLA peptide and predict the new antigen peptide. The separated peptide was sequenced by fluorescence sequencing, and the target peptide was added to the mixture to determine the detection limit.
[0151](ii) Separation and verify HLA peptide
[0152]Two single allele B cell lines (HLA-A2603 and HLA B0702) were purchased from the International Tissue Compatibility Working Group, such as the publications (Petersdorf et al., 2013). Cultivate 3 × 10 as described8A cells were carried out, and HLA peptide purification was carried out (Abelin et al., 2017). The schematic diagram of this method is likeFigure 6 Indicated in it.
[0153]The separated HLA peptide is identified by the LC coupling stringing spectrometer (SWSSPROT) using the human protein group and the use of the HLA peptide is used in the literature. Others, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap