Recombinant cd1-restricted t cells and methods
A restricted, cell-based technology for cell-based immunotherapy that addresses issues such as being unsuitable for clinical settings, complex, and difficult to obtain vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0047] The following description provides one of ordinary skill in the art with exemplary guidance for the preparation and use of the recombinant cells described herein. However, these examples should not be construed as limiting the inventive subject matter.
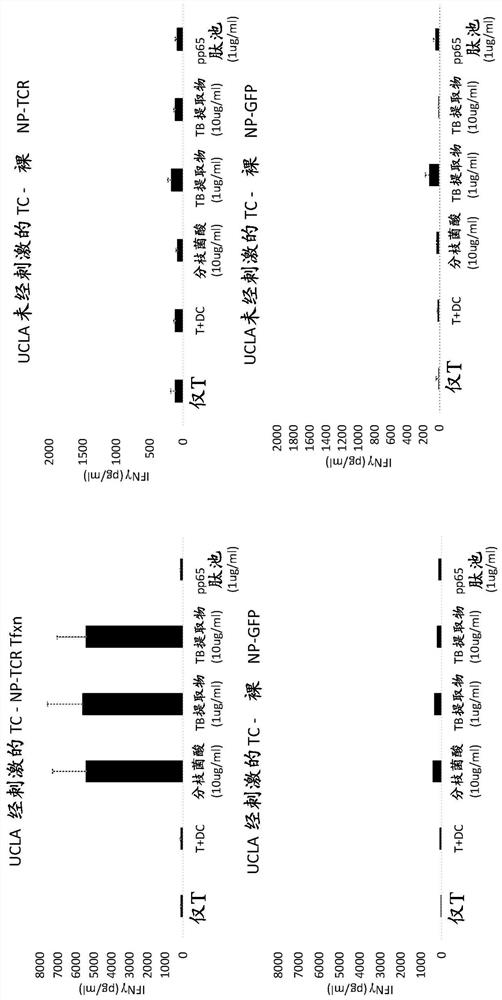
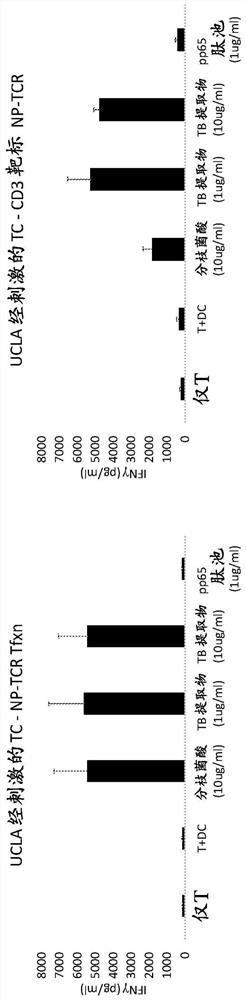
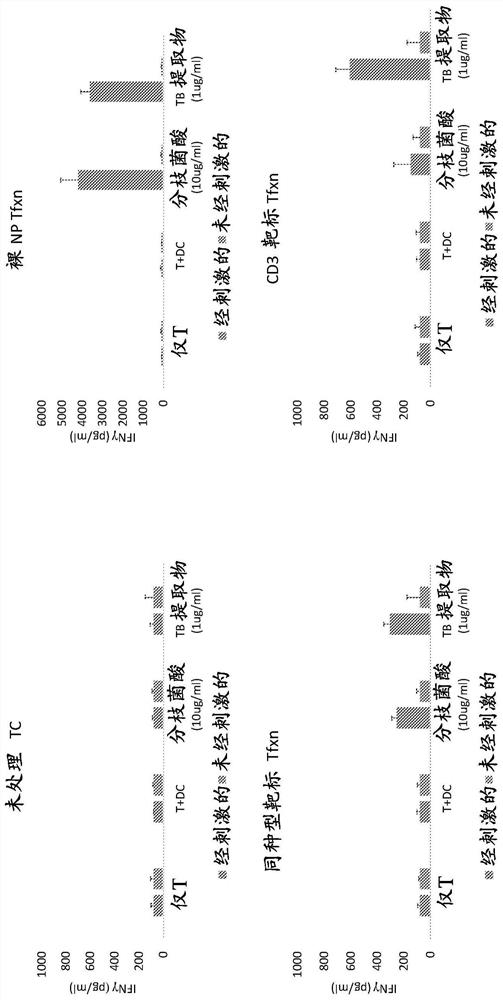
[0048] To study the effect of T cell stimulation and type of transfection on T cell responses to various antigens, 'naked' non-targeting PBAE (poly(β-amino ester)) nanoparticles associated with the following two types of RNA were transfected separately. T cells were stained: RNA encoding clone 18 TCR (bicistronic construct with clone 18 TCR alpha and beta chains) and RNA encoding GFP (green fluorescent protein). In this case, isolated T cells were cultured for 24 hours with a mixture of anti-CD3 and anti-CD28 antibodies (Immunocult Human CD3 / CD28 T Cell Activator, Stemcell Technologies) and then before transfection Stimulation was achieved by culturing for an additional 24 hours in unsupplemented medium. PBAE-RNA nano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com