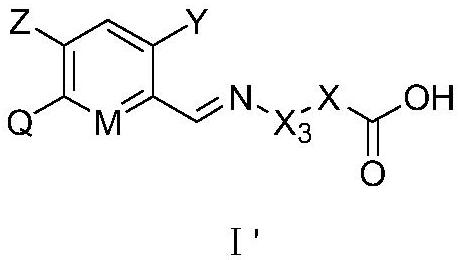
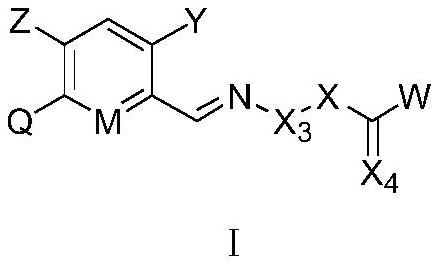
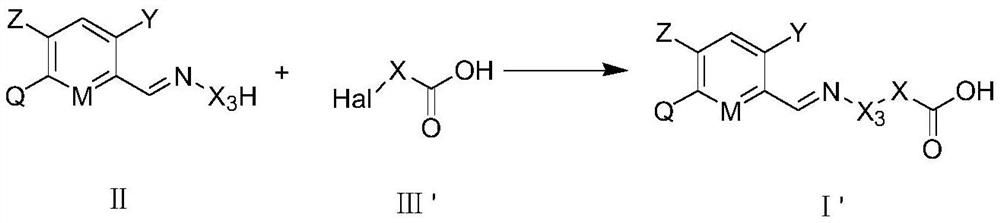
Carboxylic acid derivative substituted imino aryl compound as well as preparation method, weeding composition and application thereof
A technology of carboxylic acid derivatives and imino groups, which is applied in the field of pesticides and can solve the problem of unsatisfactory crop selectivity in the herbicidal performance of harmful plants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0164] The following examples serve to illustrate the invention and should not be construed as limiting the invention in any way. The protection scope of the present invention is described by the claims.
[0165]In view of the economy and diversity of the compounds, we preferably synthesized some compounds. Among the synthesized compounds, selected parts are listed in Table 1 and Table A below. The specific compound structures and corresponding compound information are shown in Table 1 and Table A. The compounds in Table 1 and Table A are just to better illustrate the present invention, but do not limit the present invention. For those skilled in the art, it should not be understood that the scope of the above subject of the present invention is limited to the following compounds.
[0166] Table 1 Compound structure and its 1 HNMR
[0167]
[0168]
[0169]
[0170]
[0171]
[0172]
[0173]
[0174]
[0175]
[0176]
[0177]
[0178]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com