Novel crystalline forms of an nrtti compound
A crystallization and form technology, applied in the fields of carbohydrate active ingredients, organic chemistry, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
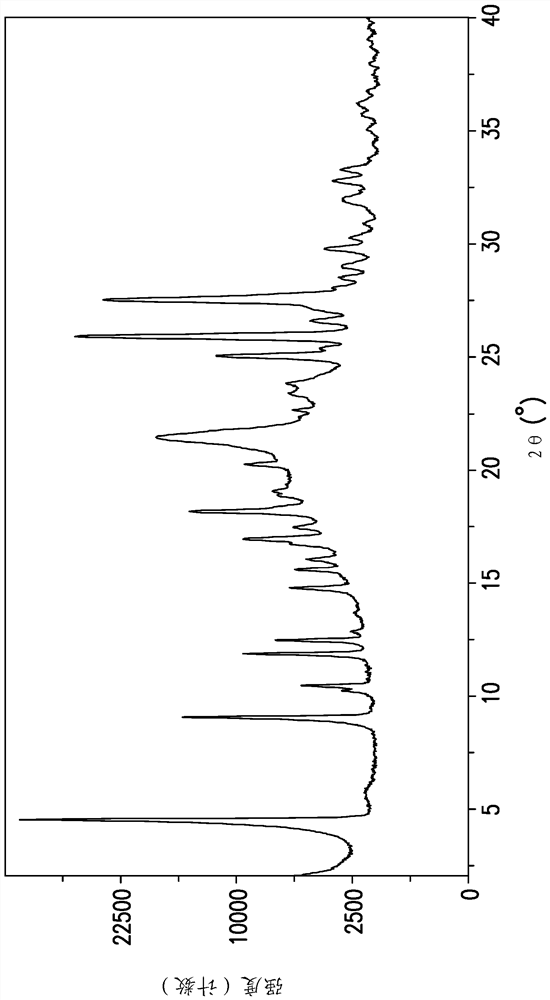
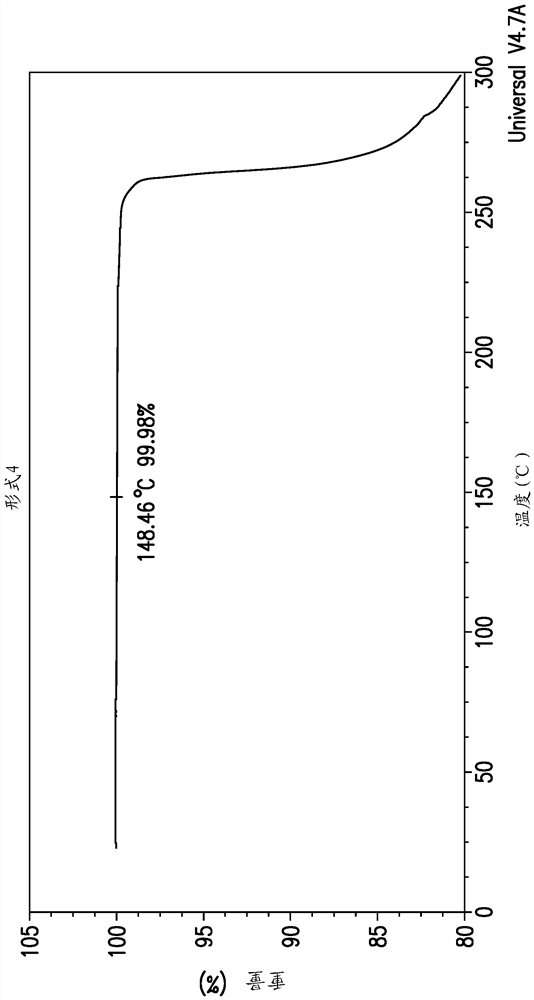
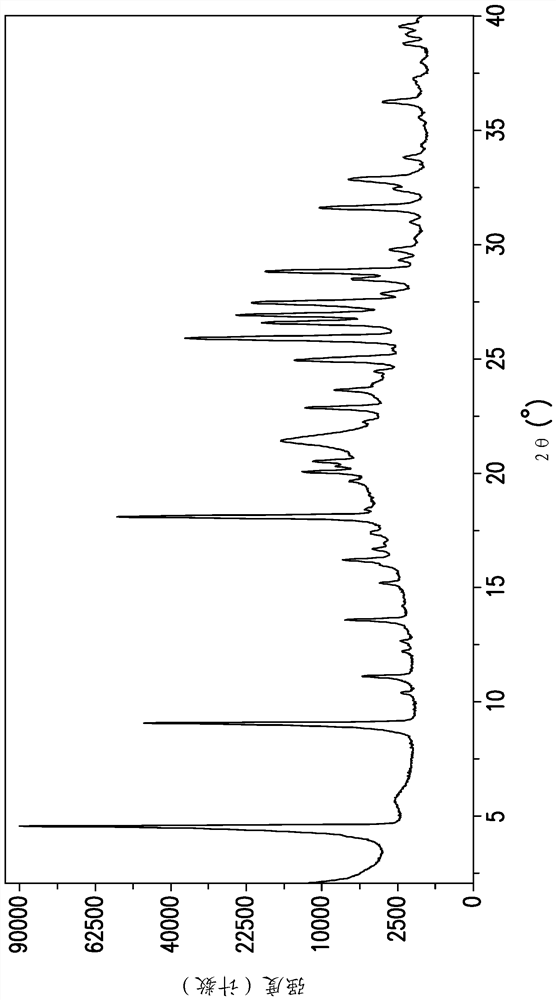
[0198] The Gibbs free energy (ΔG) of a phase is a fundamental thermodynamic parameter that determines the relative stability of a polymorph at a given temperature. The solubility of a particular polymorph is related to ΔG by the equation ΔG = -RTln(solubility). The polymorph with the lowest solubility is considered the thermodynamically stable phase at a given temperature. Table 6 lists the measured solubility of Forms 1, 2 and 4 of EFdA in acetonitrile between 25.0 and 65.0°C. The data clearly demonstrate that the most thermodynamically stable phase of EFdA is Form 4 and that Form 2 is the least stable phase in the measured temperature range. Relatively low ΔΔG values for conversion of Form 1 to Form 4 (i.e., small energy gap between Form 1 and Form 4; 0.21–0.35 kJ / mol), fast crystallization kinetics compared to Form 1 and Form 4 The combination of polymorphism and polymorphism results in the difficulty of isolating form 4 using conventional polymorphic screening methods....
Embodiment 2
[0203] Example 2: Monohydrate crystalline form MH of EFdA
[0204] Form MH of EFdA can be obtained in suitable starting amounts by the synthetic method described in US Patent No. 7,339,053.
[0205] As will be appreciated by those skilled in the art, the use of seed crystals in the preparation of Form 1 and Form 4 as described in Examples 4 and 6 is not initially required, but after making initial quantities of crystalline Form 1 and Form 4 respectively for optimal preparation.
Embodiment 3
[0206] Example 3: Anhydrous crystalline form 1 of EFdA
[0207] EFdA Form 1 was prepared by adding 1.92 grams of EFdA solid and 16.0 grams of methanol (MeOH) to a clean reactor and heating to 65 °C while stirring. The mixture was cooled to 40°C over 30 minutes, to 20°C over 1 hour, then stirred at 20°C for ~14 hours. Filter the slurry and pass through N at ambient temperature 2 The filter cake was dried by passing the filter cake for 2 hours. 1.59 g of EFdA Form 1 was collected in ~88% isolated yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com