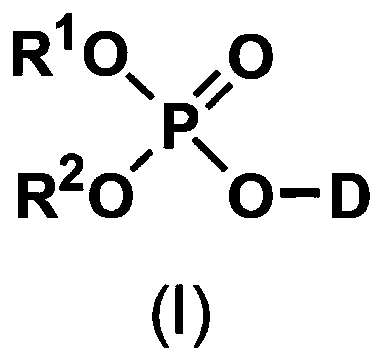
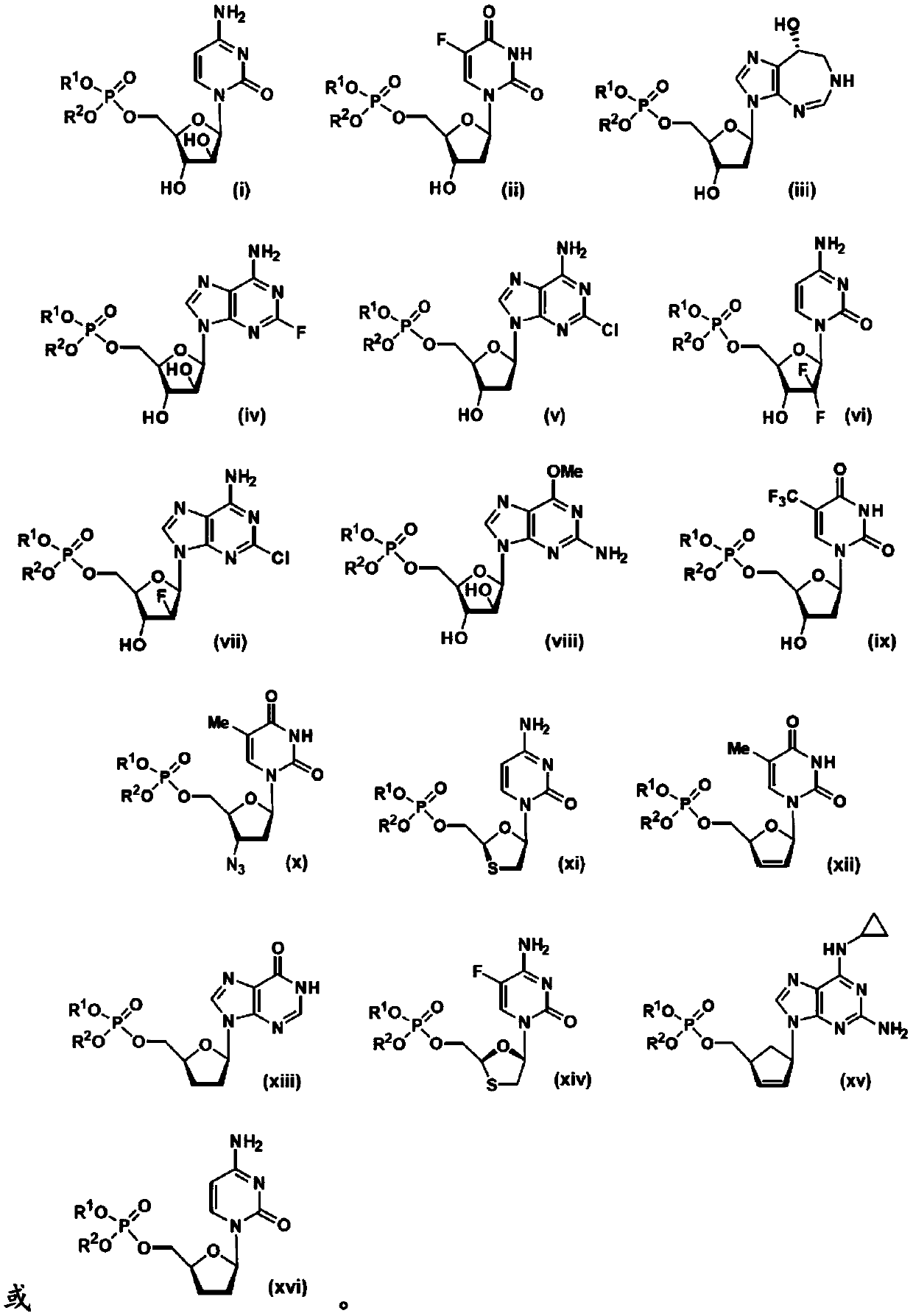
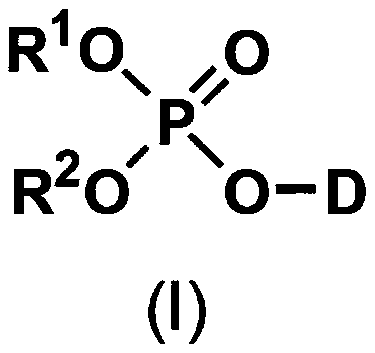
5'-position dibenzyl monophosphate derivative of nucleoside-based anticancer agent or antivirus agent
A technology of antiviral drugs and nucleosides, applied in the field of compounds, can solve the problems of low stability, high cytotoxicity of compounds, and inability to show clinical effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Activation of nucleosides with phosphorus oxychloride and subsequent condensation with benzyl alcohol
[0098] Suspend nucleosides (0.5 mM) in about 1 mL of triethyl phosphate at room temperature, add 93 μL of phosphorus oxychloride (about 2 times the raw material in terms of moles) to it under cooling to 0 ° C, and stir about 2 hours. Next, the corresponding benzyl alcohol (about 10 times by mole) and about 0.4 mL (about 10 times by mole) of pyridine were added to the solution, and stirred for another hour while cooling to 0°C. The reaction solution was poured into an ethyl acetate-water mixture, neutralized with dilute sodium bicarbonate solution, and then extracted with ethyl acetate. The extract was washed with saturated brine, and dried over anhydrous magnesium sulfate. The extract after removing the insoluble matter was dried under reduced pressure, and the obtained oily residue was separated and purified with a silica gel column (Yamazen Smart Flash MS system),...
Embodiment 2
[0100] Condensation of nucleosides and dibenzyl phosphorochloride derivatives
[0101] Suspend nucleosides (0.5mM) in 1.0mL of anhydrous pyridine at room temperature, and add about 0.25mL (about 1.2 times by mole) of the corresponding dibenzyl chlorophosphoric acid under cooling to 0°C ester derivative and stirred for about 1 hour. This reaction solution was poured into an ethyl acetate-water mixture, neutralized with dilute sodium bicarbonate solution, and then extracted with ethyl acetate. The extract was washed with saturated brine, then dried over anhydrous magnesium sulfate, the extract from which insoluble matter was removed was dried under reduced pressure, and the obtained oily residue was separated and purified with a silica gel column (Yamazen Smart Flash MS system) , thereby obtaining a 5'-position dibenzyl monophosphate derivative of a nucleoside as the target compound. This method is referred to as Synthetic Method B hereinafter.
[0102] The following shows th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com