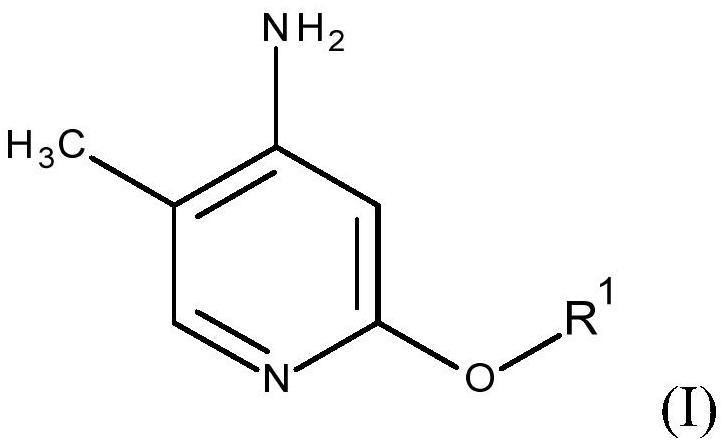
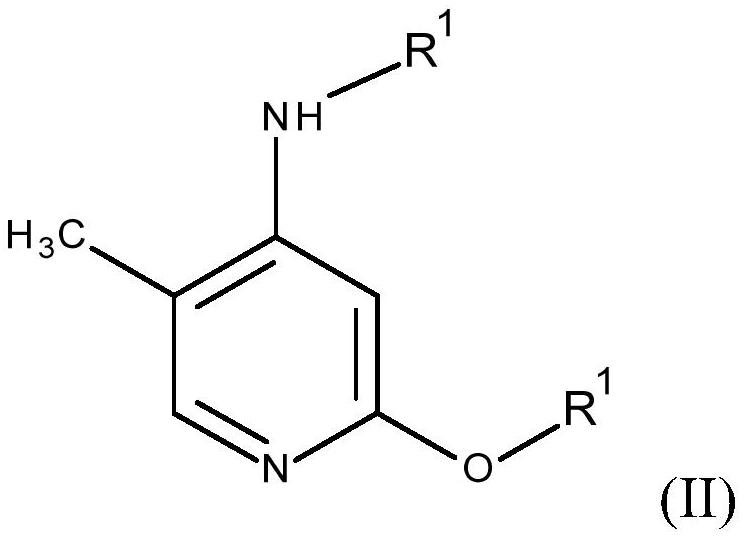
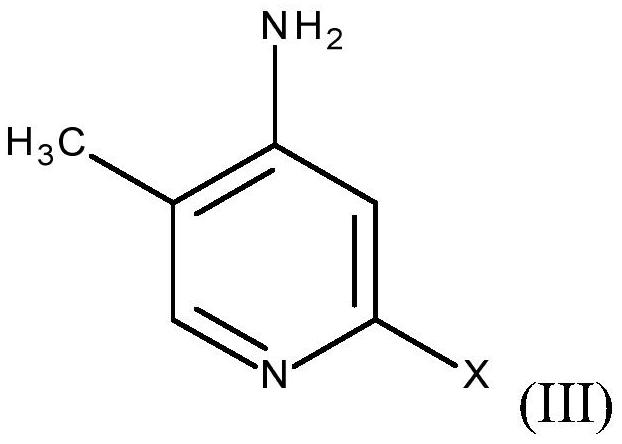
Process for preparing 2-alkoxy-4-amino-5-methyl-pyridines and/or 2-alkoxy-4-alkylamino-5-methyl-pyridines
A technology of alkyl and cycloalkyl, applied in the field of compounds produced by it, can solve the problem of no 2-alkoxy-4-amino-5-picoline, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0108] Example 1a: Preparation of 4-amino-2-benzyloxy-5-picoline / 4-benzylamino-2-benzyloxy-5-picoline (the present invention)
[0109] A mixture of 60 g (0.55 mol) of benzyl alcohol and 40 g (0.22 mol) of a 30% methoxide sodium methoxide solution was heated to about 150° C. and the resulting methanol was distilled off. After reaching 150° C., a vacuum of 90 mbar was applied with continuous removal of distillate, and the mixture was stirred under these conditions for a further 1 hour. Subsequently, the vacuum was increased at 150° C. until distillation started. Distillation was continued under these conditions until the maximum temperature reached or exceeded 100°C.
[0110] At 150° C. and under standard pressure, a solution of 10 g (0.07 mol) of 4-amino-2-chloro-5-methylpyridine in 20 g (0.18 mol) of benzyl alcohol was then metered in over 2 hours. After metering, the reaction mixture was further stirred at 150° C. until conversion was complete.
[0111] After cooling to r...
example 3
[0146] Example 3: Preparation of 4-amino-2-propoxy-5-methylpyridine (the present invention)
[0147] In an autoclave, a mixture of 24 g (0.17 mol) of 4-amino-2-chloro-5-picoline, 120 g (2.0 mol) of n-propanol and 25.8 g (0.65 mol) of sodium hydroxide was heated to 145°C, and the mixture was stirred at these conditions for 24 hours.
[0148] After cooling to room temperature, 150 g of water were added to the reaction mixture, which was freed from alcohol by distillation at standard pressure to a minimum temperature of about 110°C. 90 g of toluene were added to the distillation bottoms and controlled at a temperature of about 50°C. At this temperature the aqueous phase separates out.
[0149] After adding another 90 g of water, the resulting mixture was adjusted to pH 8-9 with 30% aqueous hydrochloric acid. Subsequently, the toluene was separated off at standard pressure, and 12 g of isopropanol were added to the remaining suspension at about 70° C. After cooling to room t...
example 4
[0151] Example 4: Preparation of 4-amino-2-isopropoxy-5-picoline (present invention)
[0152] In an autoclave, a mixture of 24 g (0.17 mol) of 4-amino-2-chloro-5-picoline, 120 g (2.0 mol) of isopropanol and 25.8 g (0.65 mol) of sodium hydroxide was heated under spontaneous pressure to 145°C, and the mixture was stirred at these conditions for 24 hours.
[0153] After cooling to room temperature, 100 g of water were added to the reaction mixture, which was freed from alcohol by distillation at standard pressure to a minimum temperature of about 110°C. 90 g of toluene were added to the distillation bottoms and controlled at a temperature of about 50°C. At this temperature the aqueous phase separates out.
[0154] After adding another 90 g of water, the resulting mixture was adjusted to pH 8-9 with 30% aqueous hydrochloric acid. Subsequently, the toluene was separated off at standard pressure, and 12 g of isopropanol were added to the remaining suspension at about 70° C. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com