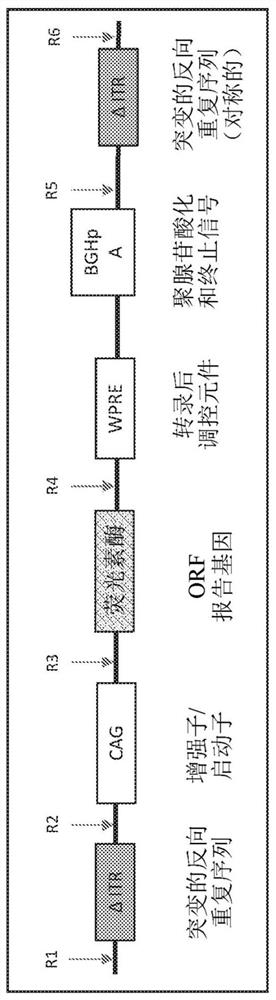
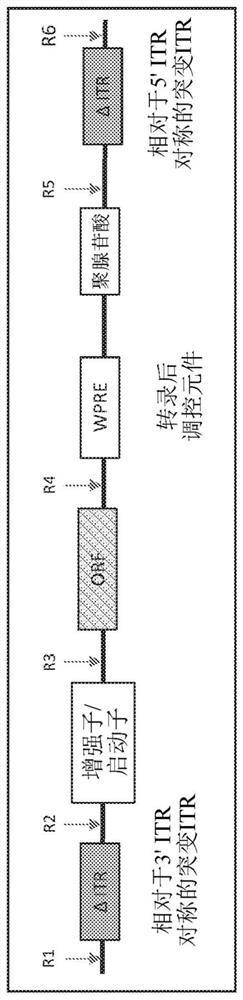
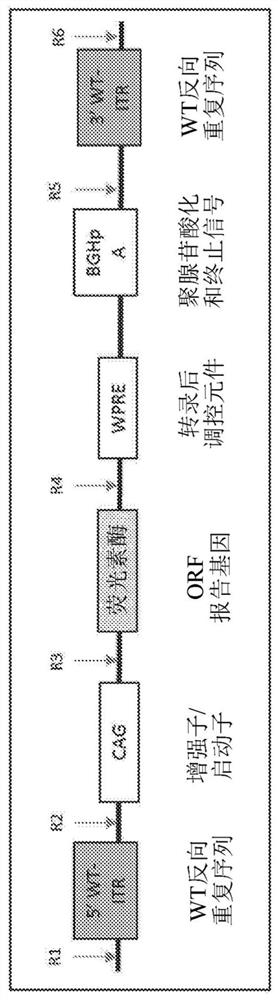
Modified closed-ended DNA (CEDNA) comprising symmetrical modified inverted terminal repeats
A closed-end, symmetric technology that can be used in the field of gene therapy to solve problems such as limited viral packaging capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0564] The following examples are offered by way of illustration and not limitation.
example 1
[0565] Example 1: Construction of ceDNA vector
[0566] The use of polynucleotide construct templates to generate ceDNA vectors is described in Example 1 of PCT / US18 / 49996. For example, the polynucleotide construct template used to generate the ceDNA vectors of the invention can be a ceDNA-plasmid, ceDNA-bacmid and / or ceDNA-baculovirus. Without being bound by theory, in a permissive host cell, in the presence of, for example, Rep, a polynucleotide construct template having two symmetrical ITRs and an expression construct is replicated to generate a ceDNA vector, wherein at least one of the ITRs is relative to The wild-type ITR sequence is modified. ceDNA vector generation goes through two steps: first, the template is cleaved ("rescued") from the template backbone (e.g., ceDNA-plasmid, ceDNA-bacmid, ceDNA-baculovirus genome, etc.) by the Rep protein; and second, the cleavage The ceDNA vector under the Rep-mediated replication.
[0567] One exemplary method of generating a c...
example 2
[0595] Example 2: Generation of synthetic ceDNA from double-stranded DNA molecules by excision
[0596] Synthetic production of ceDNA vectors is described in Examples 2-6 of International Application PCT / US19 / 14122, filed January 18, 2019, which is incorporated herein by reference in its entirety. One exemplary method of producing ceDNA vectors using synthetic methods involves excision of double-stranded DNA molecules. Briefly, ceDNA vectors can be generated using double-stranded DNA constructs, see e.g. PCT / US19 / 14122 Figure 7A -8E. In some embodiments, the double-stranded DNA construct is a ceDNA plasmid, see, eg, Figure 6 of International Patent Application PCT / US2018 / 064242 filed December 6, 2018).
[0597] In some embodiments, a construct for making a ceDNA vector comprises a regulatory switch as described herein.
[0598] For purposes of illustration, Example 1 describes the generation of a ceDNA vector, an exemplary closed DNA vector generated using this method. Ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com