Methods and compositions for identifying whether a subject suffering from a cancer will achieve a response with an immune-checkpoint inhibitor
An immune checkpoint, subject technology, applied in the field of oncology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
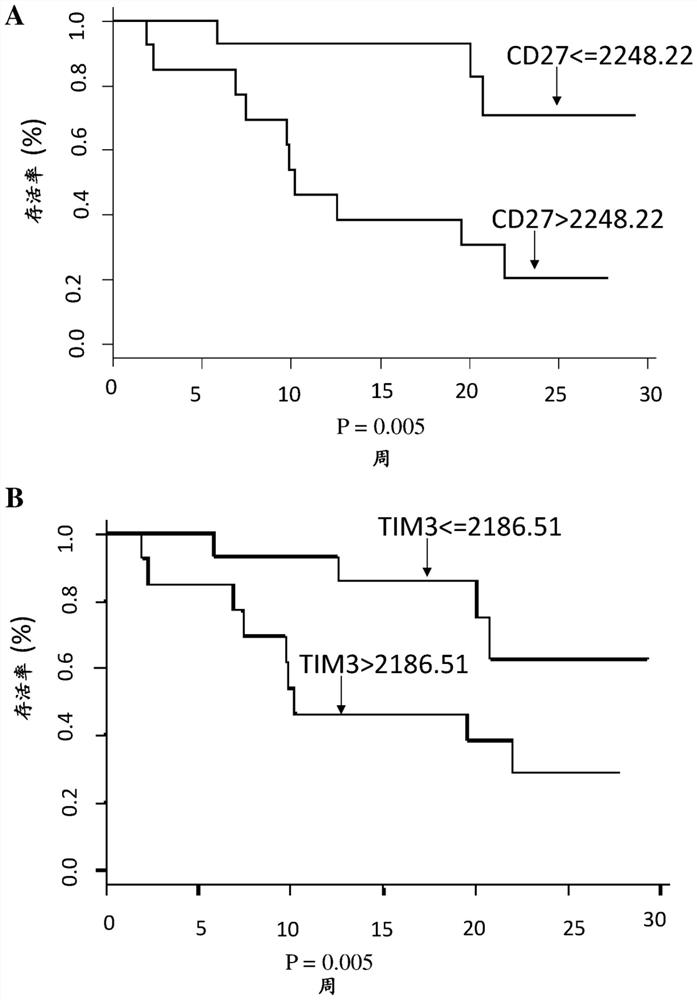
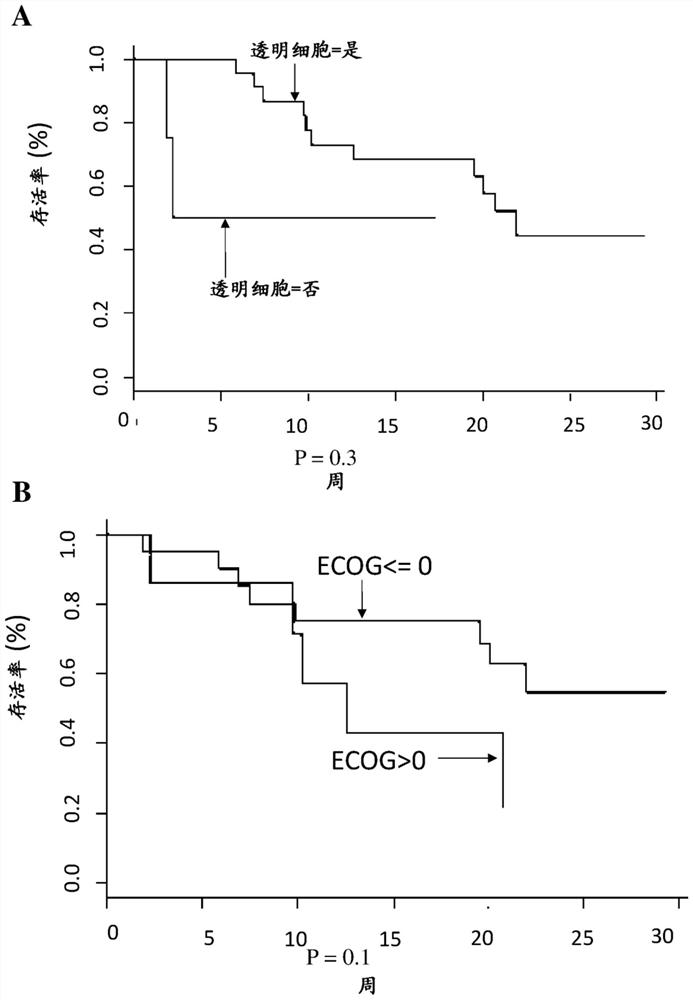
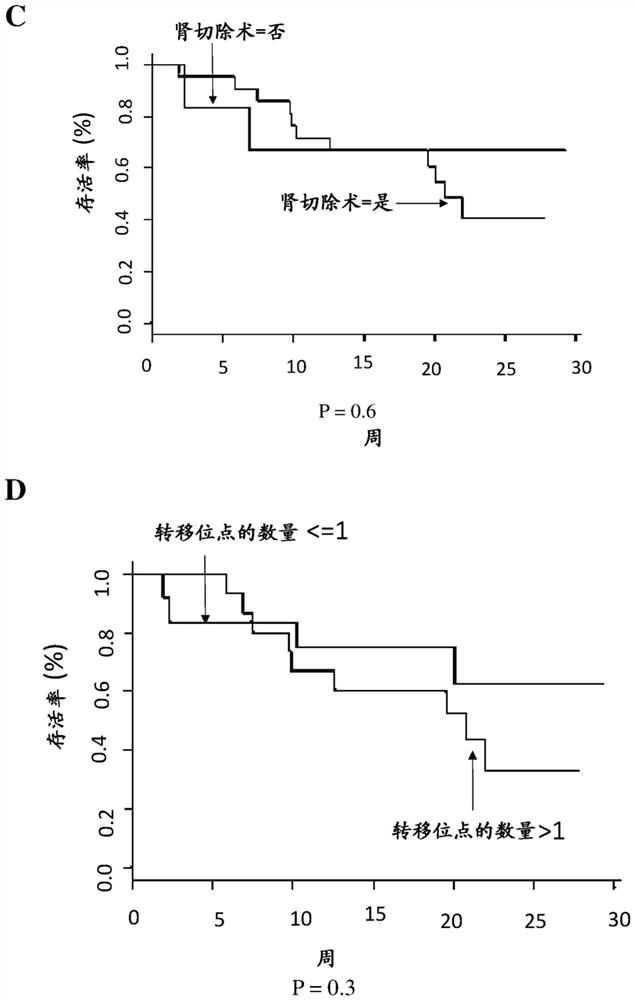
[0007] The inventors have worked with two cohorts of patients: 1) the checkpoint cohort (CPP: 2015-08-04-MS2) established at Georges Pompidou Europea Hospital, including all patients who received immunotherapy (anti-PD-1 / PD-L1) treated patients, and blood and tissue samples were collected after obtaining the patient's consent. Twenty-seven renal cell carcinoma patients from this cohort were included in this study. Twenty-two of the patients had clear cell renal carcinoma. 2) Another set of specimens came from the Preinsut clinical study, in which patients with renal cell carcinoma received two cycles of sunitinib before nephrectomy [8]. Twenty-seven patients from this cohort were also included in this study.
[0008] The inventors have identified a soluble marker, CD27, present in the plasma of renal cell carcinoma patients, whose pretreatment concentrations were predictive of anti-PD-1 / PD-L1 responses. This marker is more like a predictive marker of response to anti-PD1 / P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com