Novel anti-vegfr2 monoclonal antibody and its preparation and application
A monoclonal antibody, a new type of technology, applied in the field of bioengineering, can solve the problems of low yield and difficult assembly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1, construction and screening of antibody library
[0070] 1. Construction of antibody library
[0071] The single-chain antibody library used in the present invention is a human natural antibody library, and its main construction process is:
[0072] (1) Isolate B lymphocytes from peripheral blood or spleen, lymph nodes and other tissues, extract mRNA and reverse transcribe it into cDNA;
[0073] (2) Design antibody heavy chain and light chain primer sequences according to existing antibody gene sequence libraries (such as Kabat database, V-base, IMGT, etc.) (heavy chain primer sequences are shown in Table 1; light chain primer sequences are shown in Table 2 shown), different VH and VK gene fragments are amplified by PCR technology, and spliced into a full-length scFv single-chain antibody through the linker region;
[0074] Table 1. Primers for amplifying the heavy chain variable region VH
[0075]
[0076] K=G / T, M=A / C, R=A / G, S=G / C, W=A / T
[0077] T...
Embodiment 2
[0089] Example 2, Cloning and Expression of Antibodies
[0090] 1. Antibody cloning and transfection
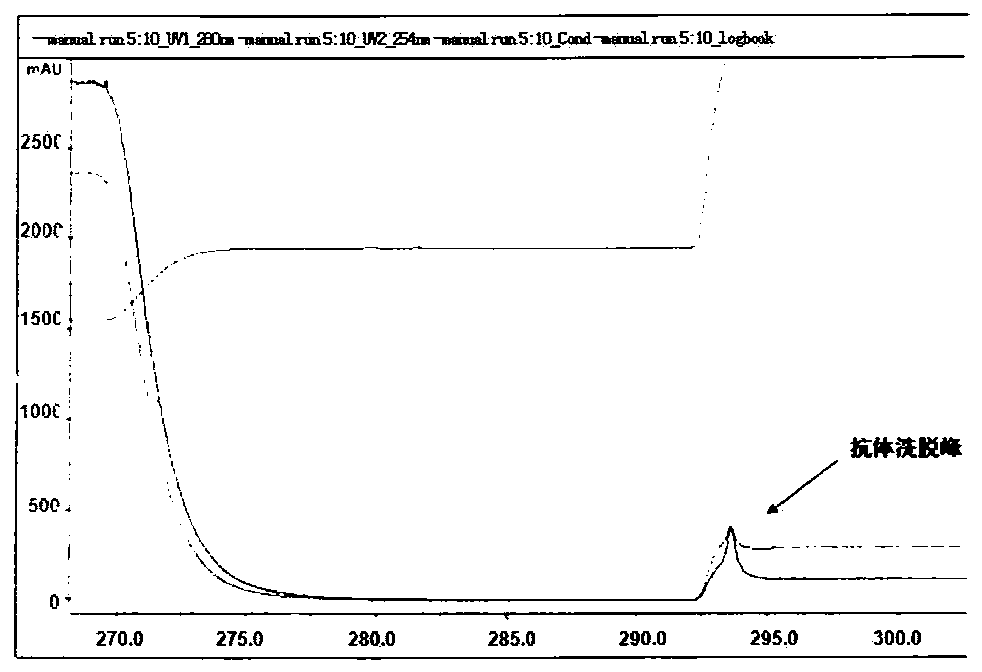
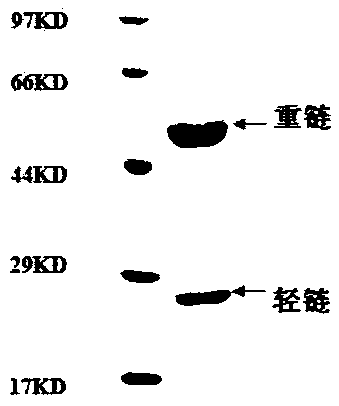
[0091] The screened variable region sequence consisting of SEQ ID NO: 1-12 was cloned into the FC fusion eukaryotic expression vector pFUSEIgG1FC or pSNEO to express the full-length antibody by conventional gene cloning method. The correct insertion of the antibody gene was identified by enzyme digestion and sequencing, followed by transfection and antibody secretory expression.
[0092] The day before transfection, digest 1×10 5 The 293 cells / mL cell density were inoculated in 6-well plates and cultured overnight until the confluence of 50-80% was optimal. The culture medium was incubated in a water bath at 37°C for 30 minutes, 200 μL serum-free medium was added to each of the 2 eppendorf test tubes, 1-2 μg DNA and 2 μl or 4 μl PEI (polyethyleneimine polymer) working solution (DNA / PEI = 1:2), mix well and incubate at room temperature for 5 minutes, quickly add the PEI dil...
Embodiment 3
[0098] Example 3, Identification of Biochemical and Biological Functions of Antibodies
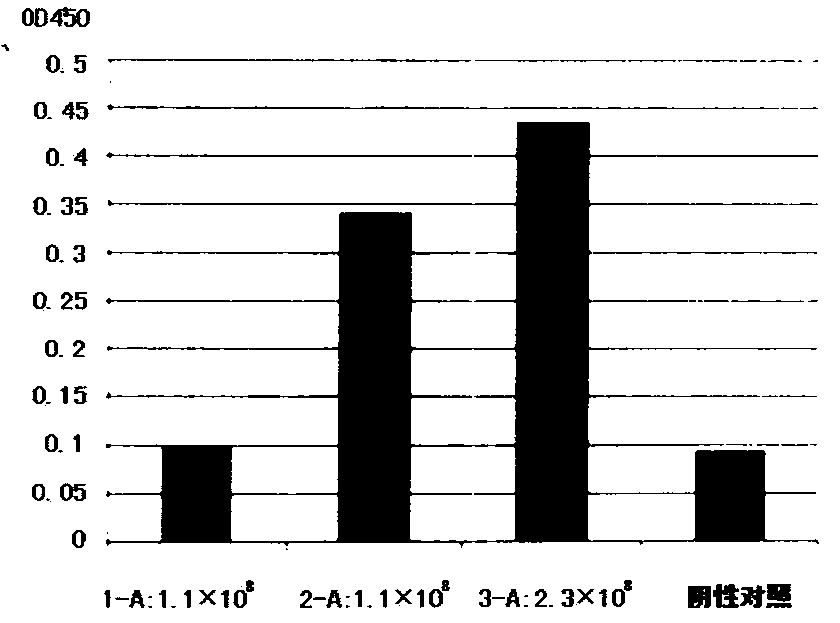
[0099] 1. Antibody binding specificity detection
[0100] The binding specificity of the antibodies was determined using the ELISA method described below. Dilute VEGFR2 or other recombinant proteins with coating buffer to a final concentration of 1 μg / ml, 100 μl / well, and incubate overnight at 4°C. Discard the coating solution, wash the wells with PBS three times, and pat dry on a clean paper towel. Add not less than 300 μL / well of blocking solution, and incubate at 37°C for 2 hours. The blocking solution was discarded, and the wells were washed three times with PBST (phosphate buffered saline, 0.05% Tween). Take 300 μL of cell supernatant after transfection for 48 hours, centrifuge at 1000 r / pm for 5 minutes, and take 100 μL / well of supernatant. Incubate at 37°C for 1h. The sample solution was discarded and washed 3 times with PBST. The detection antibody was diluted with blocking s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com