Combination therapy with a raf inhibitor and a cdk4/6 inhibitor for use in the treatment of cancer
A technology for inhibitors and cancer, applied in the field of drug combinations, can solve problems such as off-target toxicity and unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
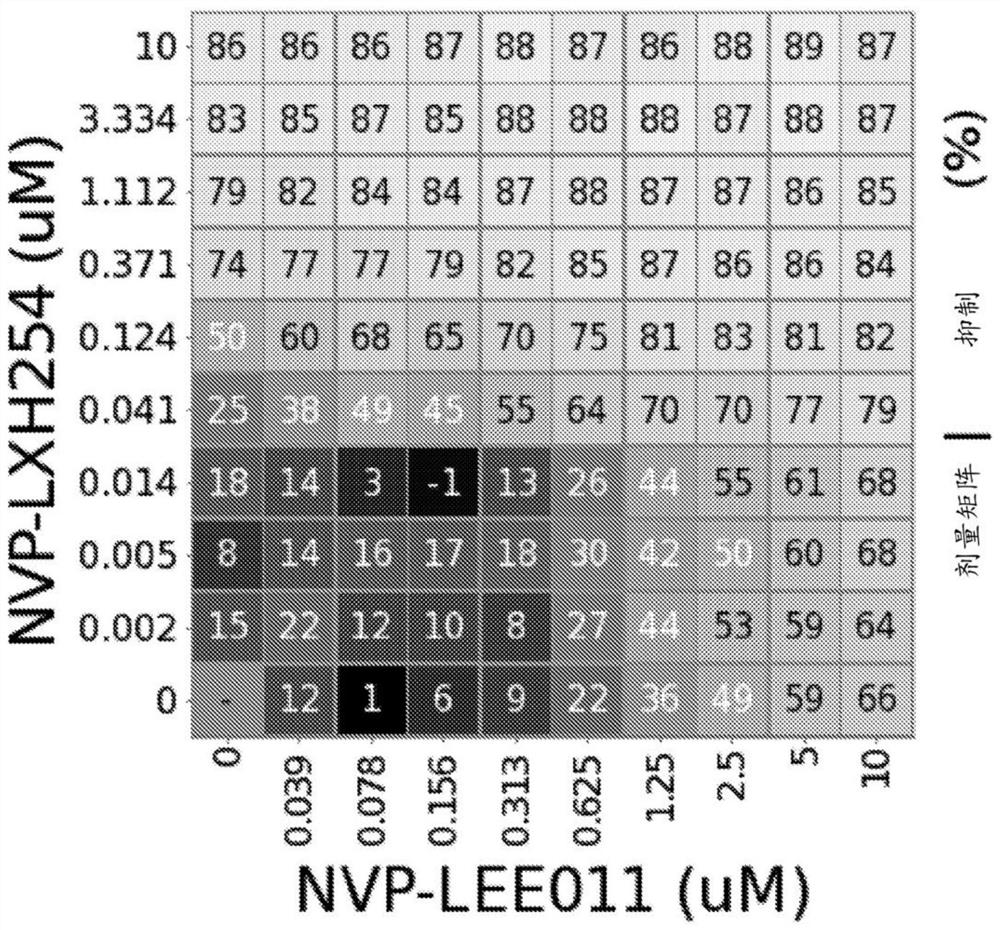
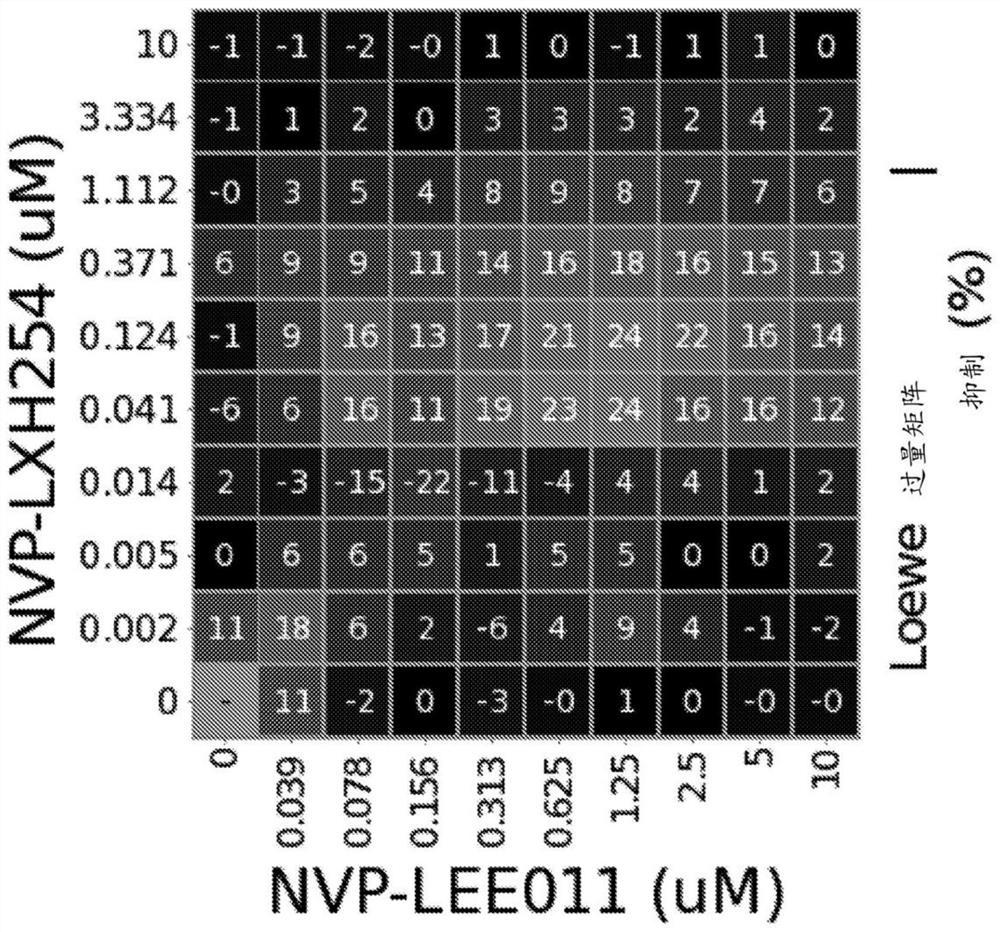
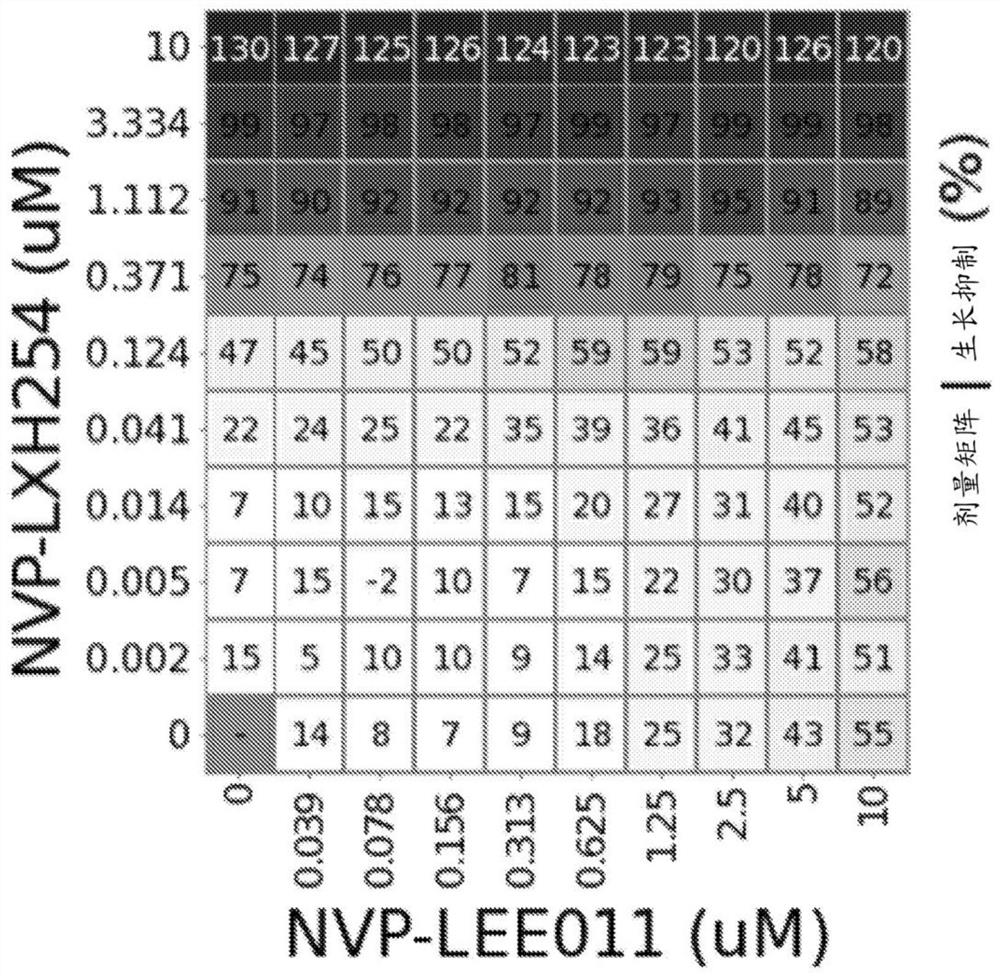
[0177] Example 1: Combination of RAF Inhibition and Enhancement of CDK4 / CDK6 Inhibition in NRAS Mutant Melanoma Cell Lines
[0178] method
[0179] A compound of formula (I) (NVP-LXH254) and ribociclib (NVP-ribociclib) were synthesized, and a compound stock solution of ribociclib was prepared in DMSO at a final concentration of 10 mM. For combination assays, the working stock was serially diluted in appropriate cell culture media in 3-fold increments (2-fold increments for ribociclib) to achieve final assay concentrations: NVP-LXH254 ranged from 10 μM to 1.5 nM, 10 μM to 39 nM (succinate form) for NVP-ribociclib.
[0180] SK-MEL-2 cells were purchased from American Type Culture Collection (ATCC), MEL-JUSO cells were purchased from German Microbiology and Cell Culture Series Co., Ltd. (DSMZ), MM415 cells were purchased from Australian Cell Bank, and IPC-298 and SK- MEL-30 cells were obtained from GNF. IPC-298, MEL-JUSO, MM415, and SK-MEL-30 cells were cultured in RPMI medium...
example 2
[0187] Example 2: Combination Efficacy of Compounds of Formula (I) and Ribociclib in Xenografts Derived from NRAS Mutant Melanoma Patients
[0188] The effect of selective dual RAF and CDK4 / 6 inhibition in vivo was investigated by combining a compound of formula (I) and ribociclib in nine xenografts derived from NRAS mutant melanoma patients.
[0189] method
[0190] Animal and Maintenance Conditions: At least 3 days prior to manipulation, outbred athymic (nu / nu) female mice (Athymic Nude-nu") (Charles River, Indianapolis, Indianapolis) were allowed ad libitum access to food and water. The Novartis NIBR animal facility was adapted to the new environment (Table 1).
[0191] Table 1 Animal characteristics
[0192]
[0193] Statement on animal welfare: Animals were handled in accordance with Novartis NIBR ACUC regulations and guidelines.
[0194] Test compounds and formulations: Compounds of formula (I) (free base form) in MEPC4 vehicle (45% Cremophor RH40+27% PEG400+18% co...
example 3
[0225] Example 3: Phase Ib, open-label, multicentre study of a compound of formula (I) in combination with ribociclib in patients with NRAS-mutant melanoma
[0226] The purpose of this study was to characterize the safety and tolerability of the dual combination of a compound of formula (I) and ribociclib in patients with NRAS-mutant melanoma and to determine the recommended dose.
[0227] The primary endpoint is
[0228] (1) Safety: Incidence and severity of adverse events (AEs) and serious AEs (SAEs), including changes in laboratory values, vital signs, and electrocardiogram (ECG), dose restrictions during cycle 1 (dose escalation only) The incidence and nature of toxicity (DLT),
[0229] (2) Tolerance: dose interruption, reduction and dose intensity.
[0230] Secondary objectives and endpoints are:
[0231] (a) Evaluation of the initial antitumor activity of a compound of formula (I) in combination with ribociclib. Overall response rate (ORR), disease control rate (DCR)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com