Amyloid precursor protein as a diagnostic marker for biliary atresia
A technique for biliary atresia, amyloid, applied in assessing the likelihood of adverse outcomes in infants, diagnosing biliary atresia or assessing risk areas for biliary atresia, capable of addressing issues such as failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
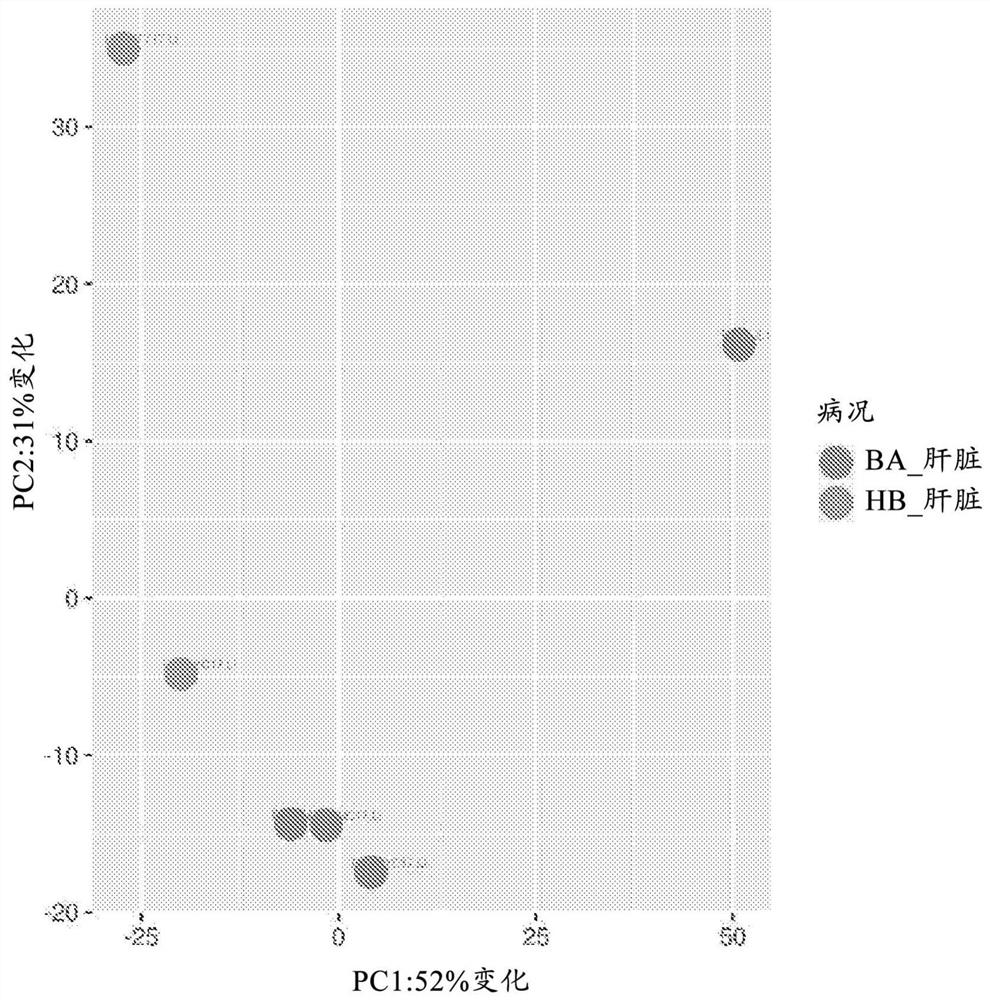
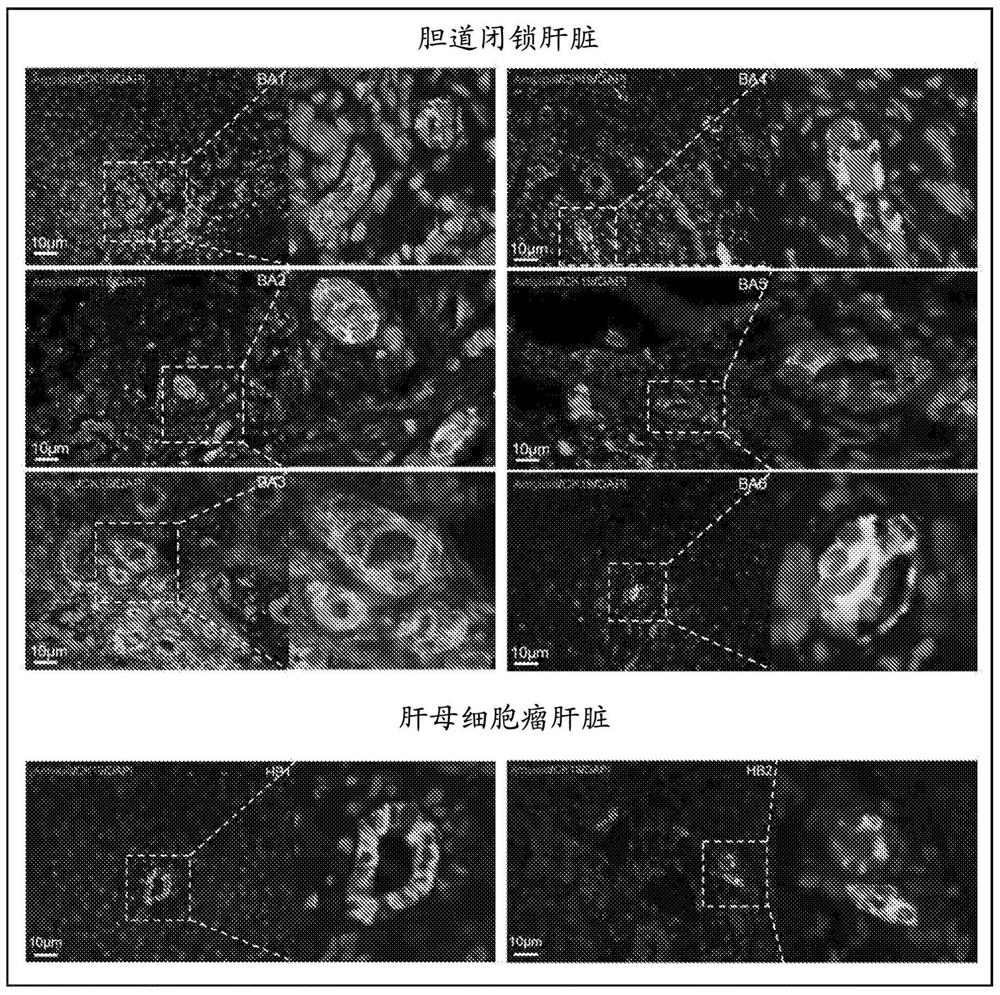
Image
Examples
Embodiment 1
[0091] Example 1: Expression of amyloid precursor protein in biliary atresia
[0092] This study reveals for the first time the specific expression of amyloid precursor protein and / or its processed form in the bile ducts of the liver of patients with biliary atresia (BA). This finding enables the design of new and reliable diagnostic methods for the early detection of BA as well as for predicting the outcome of BA treatments (eg, Kasai procedure).
[0093] introduce
[0094] Biliary atresia (BA [OMIM 210500]) is characterized by progressive fibro-obliterative cholangiopathy (disease of the bile ducts) affecting the intrahepatic and extrahepatic bile ducts and leading to obstruction of bile flow, cholestasis, and neonatal jaundice. Some infants develop BA between 2 and 6 weeks after birth. Symptoms of BA usually manifest between 2 and 6 weeks after birth because infants with BA develop progressive cholestasis, a condition in which the liver fails to excrete biliary red as bile...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com