Three-dimensional epitope of hepatitis b surface antigen and antibody binding specifically thereto
A surface antigen, hepatitis B technology, applied in the direction of antibody medical components, virus antigen components, specific peptides, etc., to achieve the effect of eliminating hepatitis B virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0070] Preparation Example 1. Preparation of an antibody specifically binding to HBsAg
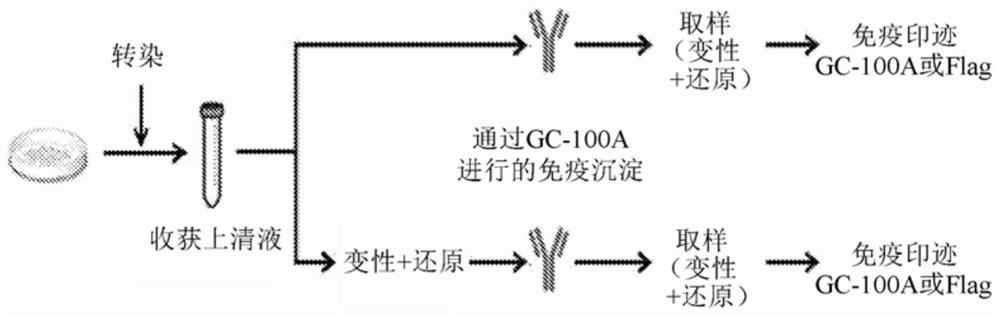
[0071] In the present invention, as the antibody specifically binding to HBsAg, a monoclonal antibody produced by a strain with accession number KCTC13760BP was used. In addition, for convenience, the monoclonal antibody produced by this strain was named GC-100A.
[0072] Experimental method 1. Native agarose gel electrophoresis (NAGE)
[0073] Huh-7 cells were transfected with empty (mock) vector or Flag-small HBsAg expression plasmid using Lipofectamine 3000. After 2 days, cells were lysed using RIPA buffer (Thermo Fisher, 89901). Centrifugation was performed at 4°C and 12,000 rpm for 15 minutes, and then the pellet-free supernatant was transferred to a new Eppendorf tube. To this was added 6X dye-loaded agarose (50% glycerol, 0.1% BPB) to a final IX concentration and mixed well. A 1.2% TBE agarose gel was prepared and the samples mixed with the dye-loaded agarose were loaded on it. ...
experiment example 1
[0087] Experimental example 1. Identification of the HBsAg epitope of GC-100A antibody
experiment example 11
[0088] Experimental Example 1.1. Identification of Possibility of Detection of HBsAg VLP Using GC-100A
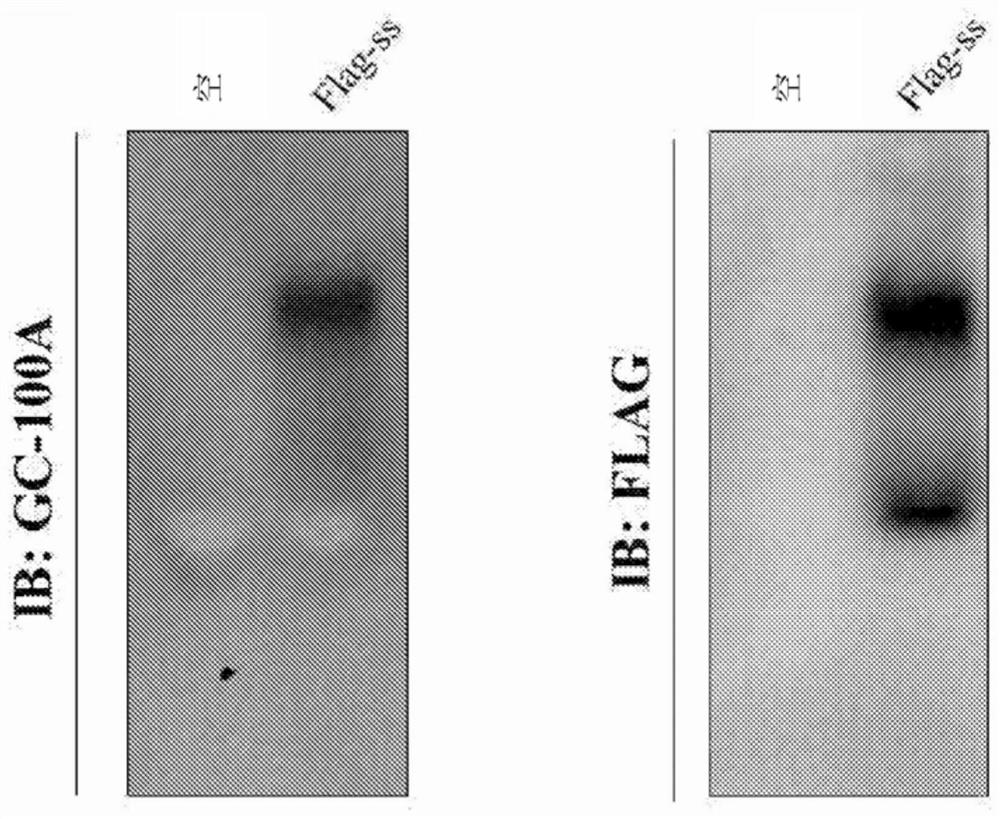
[0089] Until 2016, ELISA was the only test that made it possible to detect HBsAg with GC-100A. In addition, it was not possible to detect HBsAg with GC-100A, which is considered to have a conformational epitope, in Western blot analysis using SDS-PAGE. Therefore, a technique for detecting HBV capsid in a native state was devised and an attempt was made to detect HBsAg VLP (consisting of 100 single HBsAg molecules) using NAGE disclosed in Experimental Method 1. Since NAGE is a method for identifying macromolecules without causing protein denaturation, this method makes it possible to detect HBsAg VLPs with an anti-Flag antibody with a linear epitope as well as with GC-100A with a conformational epitope ( figure 2 ).
[0090] There was a difference between the HBsAg VLP pattern detected by GC-100A and anti-Flag antibody. In each of the upper strips, the VLP can be said to...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap