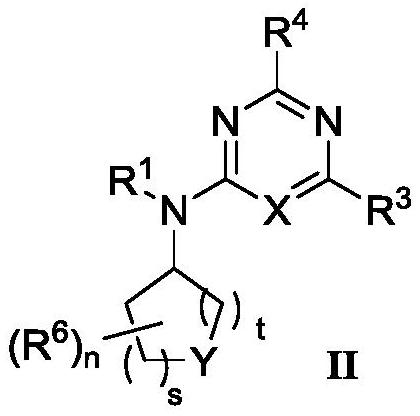
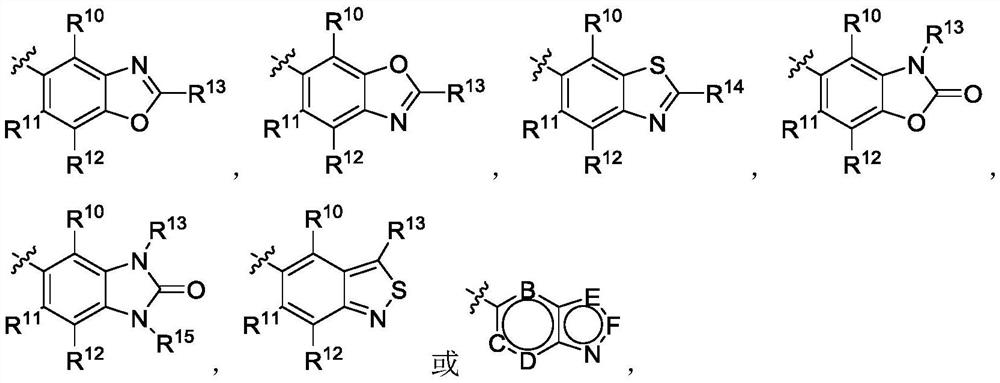
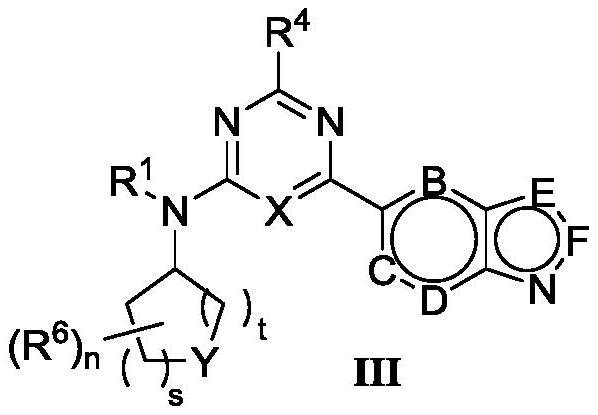
4-aminopyrimidine or 2-aminotriazine compound and preparation method thereof
A compound, alkyl technology, applied in the field of medicine, can solve problems such as action limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153]
[0154] 6-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)-N-(1-(methylsulfonyl)piperidin-4-yl)pyrimidine -4-amine
[0155]
[0156] first step
[0157] 6-Chloro-N-(1-(methylsulfonyl)piperidin-4-yl)pyrimidin-4-amine 1c
[0158] Dissolve 2,4-dichloropyrimidine 1a (0.2g, 1.3mmol) and 1-(methylsulfonyl)piperidin-4-amine 1b (0.26g, 1.5mmol) in 5mL DMSO, add N,N-di Isopropylethylamine (0.52g, 4.0mmol) was reacted at 90°C until the reaction was almost complete. The reaction liquid was added to 10mL ice-water mixture, filtered, washed with ice water, and the obtained solid was concentrated and dried under reduced pressure to obtain the title compound 1c ( 0.35 g, yield: 90%).
[0159] MS(ESI)m / z 291.1[M+H] +
[0160] 1 H NMR (400MHz, CDCl 3 )δ=8.37(s,1H),6.37(d,J=0.8Hz,1H),5.05(s,1H),4.04-3.88(m,1H),3.87-3.77(m,2H),2.90(dt , J=2.4, 12.4Hz, 2H), 2.83(s, 3H), 2.21-2.12(m, 2H), 1.65-1.58(m, 2H).
[0161] second step
[0162] 5-Bromo-2-chloro-N-(2,2-dimeth...
Embodiment 2
[0167]
[0168] 4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)-N-(1-(methylsulfonyl)piperidin-4-yl)- For 1,3,5-triazin-2-amine, refer to Example 1, wherein compound 2,4-dichloro-1,3,5-triazine was replaced by compound 2,4-dichloropyrimidine to obtain the aforementioned compound 2.
[0169] MS(ESI)m / z 462.2[M+H] +
Embodiment 3
[0171] (3R,4R)-4-((4-(4-fluoro-2-((R)-1-hydroxyethyl)-1-isopropyl-1H-benzo[d]imidazol-6-yl) -1,3,5-Triazin-2-yl)amino)-1-(methylsulfonyl)piperidin-3-ol
[0172]
[0173] first step
[0174] (3R,4R)-4-((4-Chloro-1,3,5-triazin-2-yl)amino)-1-(methylsulfonyl)piperidin-3-ol 3b
[0175] The compound methyl 2,4-dichloro-1,3,5-triazine (93 mg, 0.62 mmol) and N,N-diisopropylethylamine (107 mg, 0.82 mmol) were dissolved in 3 mL of N,N-di in methylformamide. Compound (3R,4R)-4-amino-1-(methylsulfonyl)piperidin-3-ol 3a (synthesized with reference to patent WO2019207463A1, 80mg, 0.41mmol) was added to the reaction solution in batches at room temperature. The reaction was carried out until TLC detected that the reaction was substantially complete. Add 20 mL of water, extract with ethyl acetate (20 mL×3), combine the organic phases, wash with saturated sodium chloride solution (20 mL), dry over anhydrous sodium sulfate, filter, collect the filtrate, concentrate the filtrate under redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com